Acetaminophen (APAP) has been formulated as immediate-, modified-, and extended-release tablets (APAP-IR, -MR, and -ER, respectively).

Avocado Fruit Peel as a Source of Antidiabetic drugs: Evidence from Molecular Docking Studies and ADMET Profiling
Diabetes mellitus is a long-term metabolic disorder characterized by persistent hyperglycemia, which can lead to various health problem if left untreated.

Monoaminergic Neurotransmitters are Bimodal Effectors of Tau Aggregation
Neurotransmitters (NTs) mediate trans-synaptic signaling, and disturbances in their levels are linked to aging and brain disorders.

Distinguishing Classes of Neuroactive Drugs Based on Computational Physicochemical Properties and Experimental Phenotypic Profiling in Planarians
Mental illnesses put a tremendous burden on afflicted individuals and society.

In vivo Performance of Amorphous Solid Dispersions Based on Water-Insoluble Versus Water-Soluble Carriers: Fenofibrate Case Study
The objective of this study is to address the unanswered question whether sustained supersaturation generated from amorphous solid dispersions (ASDs) formulated in insoluble hydrogel carriers will result in better bioavailability over that of spring-and-parachute type of dissolution profiles of ASDs formulated in water-soluble carriers
![Development of the Pyrido[2,3-d]pyrimidin-7(8H)-one Scaffold toward Potent and Selective NUAK1 Inhibitors](https://www.simulations-plus.com/wp-content/themes/simulations-plus/library/dist/img/default_square-large.jpg)
Development of the Pyrido[2,3-d]pyrimidin-7(8H)-one Scaffold toward Potent and Selective NUAK1 Inhibitors
The protein kinase NUAK1 has been implicated in various biological functions including cell adhesion, migration and proliferation.

Application of an Interdisciplinary Approach to Form Selection in Drug Development
Selecting the development form of an active pharmaceutical ingredient (API) in drug development is key to determining the final performance of the drug substance and drug product.

Simulations Plus and Enabling Technologies Consortium Announce Strategic Collaboration to Advance GastroPlus® Enhancements
Industry partners to provide funding and data to support ACAT™ model improvements

Development of a Physiologically Based Pharmacokinetic (PBPK) Simulation Model for Nicotine
Tobacco is a major cause of chronic diseases such as lung cancer, cardiovascular disease, and chronic obstructive pulmonary disease worldwide.

Physiologically Based Pharmacokinetic and Physiologically Based Biopharmaceutic Models Research in Latin America: A Regional Level Bibliometric Analysis, 2011-2023
The landscape of scientific research in Latin America (LA), particularly in the realms of the Physiologically Based Pharmacokinetic (PBPK) and Physiologically Based Biopharmaceutic models (PBPM), is a mosaic of varied contributions, collaborations, and specializations.

Dosage Optimization Using Physiologically Based Pharmacokinetic Modeling for Pediatric Patients with Renal Impairment: A Case Study of Meropenem
The pharmacokinetics of renally eliminated antibiotics can be influenced by changes associated with renal function and development in a growing subject. Little is known about the effects of renal insufficiency on the pharmacokinetics of meropenem in pediatric subjects.
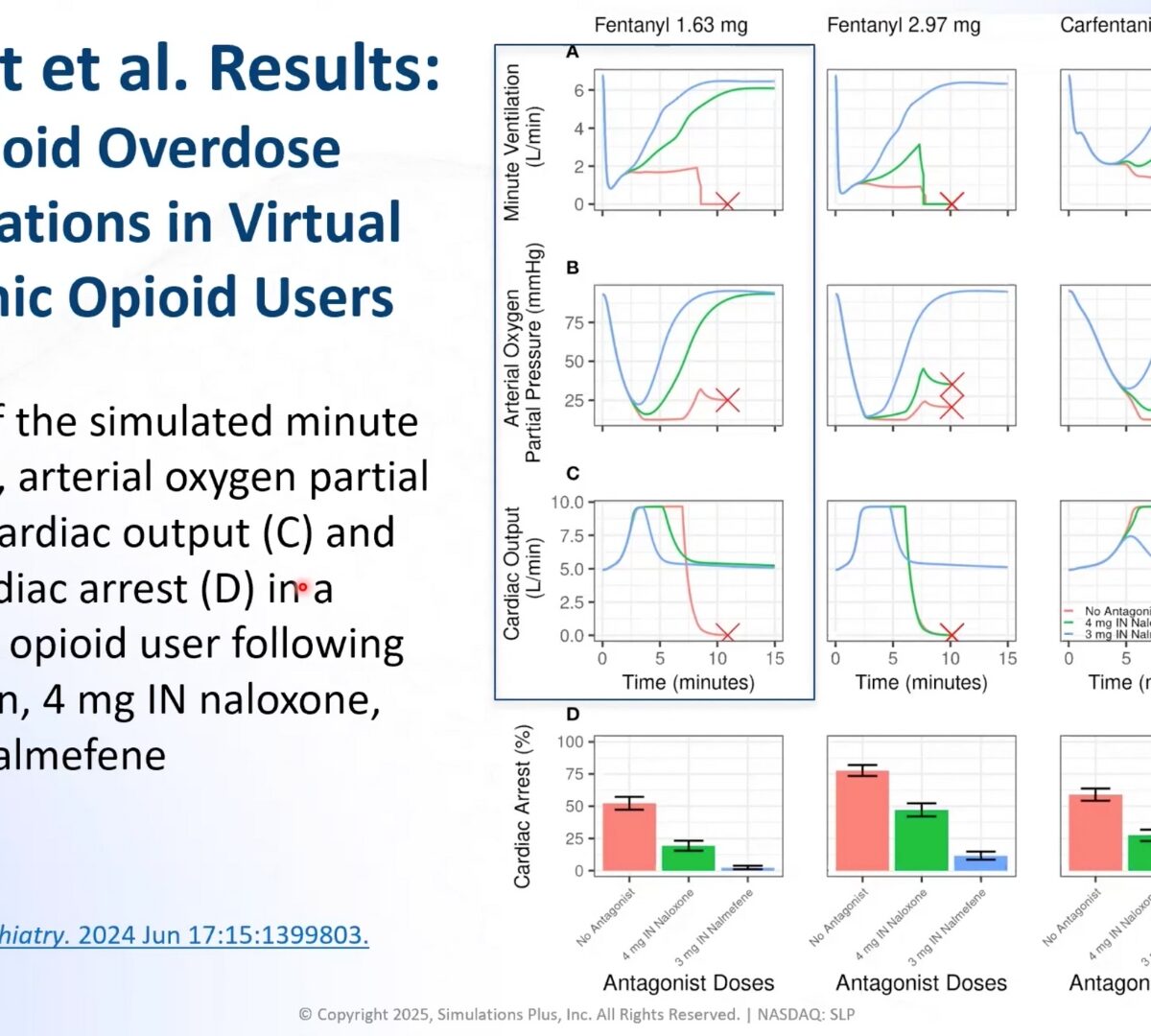
Evaluating the effectiveness of intranasal reversal agents using FDA’s translational model of opioid overdose
The number of opioid overdose deaths in the United States has continued to increase for more than two decades.

Association Between Abatacept Exposure Levels and Infection Occurrence in Patients with Rheumatoid Arthritis: Post Hoc Analysis of the AVERT-2 Study
To determine if higher serum exposure during subcutaneous (SC) abatacept treatment was associated with an increased infection risk in adult patients with early rheumatoid arthritis (RA).

Representing a Drug in a Complement QSP Model: Eculizumab as a Case Study
This blog post continues our ongoing series on leveraging quantitative systems pharmacology (QSP) to enhance drug development.

Molecular Docking, ADMET, DFT Studies, and Retrosynthesis Strategy of New Benzimidazolone Derivatives as HIV-1 Reverse Transcriptase (RT) Inhibitors
HIV-1, or Human Immunodeficiency Virus type 1, is a global pandemic that affects millions of individuals worldwide.

Simulations Plus Supported Development of Majority of FDA-Approved Drugs in 2024
SLP’s technology and expertise accelerates drug development and supports critical advancements in global healthcare

Choosing Your PK/PD Modeling Software: Key Questions About Monolix, Answered
When it comes to PK/PD modeling, many researchers stick with the tools they know, even when those tools may not be the most efficient or insightful.

Simulations Plus Reports First Quarter Fiscal 2025 Financial Results
Total revenue up 31% year-over-year primarily driven by strong software growth

Formulation Development and Evaluation, in silico PBPK Modeling and in vivo Pharmacodynamic Studies of Clozapine Matrix Type Transdermal Patches
Clozapine is a potent serotonin receptor antagonist and commonly used for the treatment of Schizophrenia.
