As a researcher in the life sciences space, you can’t measure everything as a project winds its way through the R&D process. With the data that is being generated, you’re expected to make informed decisions as quickly as possible. A wrong one sets you back severely, both in terms of time, money, and getting to market. Now, more than ever, it is important to consider incorporating new technology across your company – like PBBM & PBPK modeling.

What if it were possible to:
- Integrate the data which has been collected and, within the context of a virtual animal or human model, gain unique insights that improve your chances for success?
- Run simulations for different scenarios to avoid costly surprises?
- Utilize the outputs from these models to potentially waive expensive studies requested by regulatory agencies?
It’s possible to do this, and more, with the GastroPlus PBPK modeling platform.
GastroPlus is a mechanistically based simulation software package that simulates intravenous, oral, oral cavity, ocular, inhalation, dermal, subcutaneous, and intramuscular absorption, biopharmaceutics, pharmacokinetics, and pharmacodynamics in humans and animals. This smoothly integrated platform combines a user-friendly interface with powerful science to help you make faster and more informed project decisions! GastroPlus has been separated into modules to make it easier for companies to license only the features they need in each department.
GastroPlus® Modules
Ensuring the safety of drug therapies by leveraging our expertise in the areas of drug-induced liver injury, metabolic diseases, and modeling and simulation.
From the beginning, a conscious decision has been made to carefully implement the best theories and develop novel approaches within the GastroPlus physiologically-based pharmacokinetics (PBPK) & physiologically based biopharmaceutics modeling (PBBM) models. This dedication to science is a major reason why our predictions are consistently ranked #1 in independent comparisons. You provide the (limited) data, GastroPlus provides everything else:
- Simple, intuitive user interface
- Model customization
- High-quality plots & figures for reporting purposes
- Excellent customer support
- Incubator & Biotech Startup Program
- Thriving online GastroPlus User Group to connect users and provide an online forum for sharing best practices
- Flexible licensing models. Ask us for more details.
- Integration with our other tools. Seamlessly define inputs for your PBPK & PBBM models using the top-rated quantitative structure-activity relationship (QSAR) models from ADMET Predictor®. Strengthen the in vitro-in vivo extrapolation of dissolution & absorption inputs with DDDPlus™ and MembranePlus™. And, inform quantitative systems pharmacology (QSP) & toxicology (QST) models to predict drug-induced liver injury (DILI) or non-alcoholic fatty liver disease (NAFLD) & nonalcoholic steatohepatitis (NASH) – DILIsym® and NAFLDsym®
The in vivo processes which are considered in the GastroPlus simulations, and the methods we’ve introduced to parameterize them, are too numerous to list here – instead, take a peek at the GastroPlus datasheet for more details.
GastroPlus® is software for modeling and the conducting of simulations of properties of drugs or chemicals including absorption, release rate, bioavailability, pharmacokinetics, and pharmacodynamics; computer software which allows for prediction of drug-to-drug interactions; computer software for the prediction of drug effects on animals and virtual patient populations; computer software which allows researchers to adjust pharmacodynamic models to observed data and to then use the resulting fitted models to predict pharmacodynamic changes due to changes in dose, dosage form, and dosing regimen of a drug or chemical; computer software which allows for the generation of in vitro-in vivo correlations; computer software which allows for the prediction of absorption and systemic distribution/elimination for large molecules.
The GastroPlus PBPK modeling and simulation platform has been utilized by companies across various industries and departments since 1998. Some of the routine applications include:
- Screening compound libraries through the QSAR & PBPK marriage to prioritize in vivo testing
- Predicting first-in-human (FIH) and animal doses with best-in-class IVIVE methods
- Supporting animal or human risk assessment studies
- Simulating steady-state and dynamic drug-drug interactions (DDI) for regulatory submissions
- Building PBPK, PBBM, PD models to estimate efficacious dose levels
- Assisting with Quality by Design (QbD) implementation to define product specifications
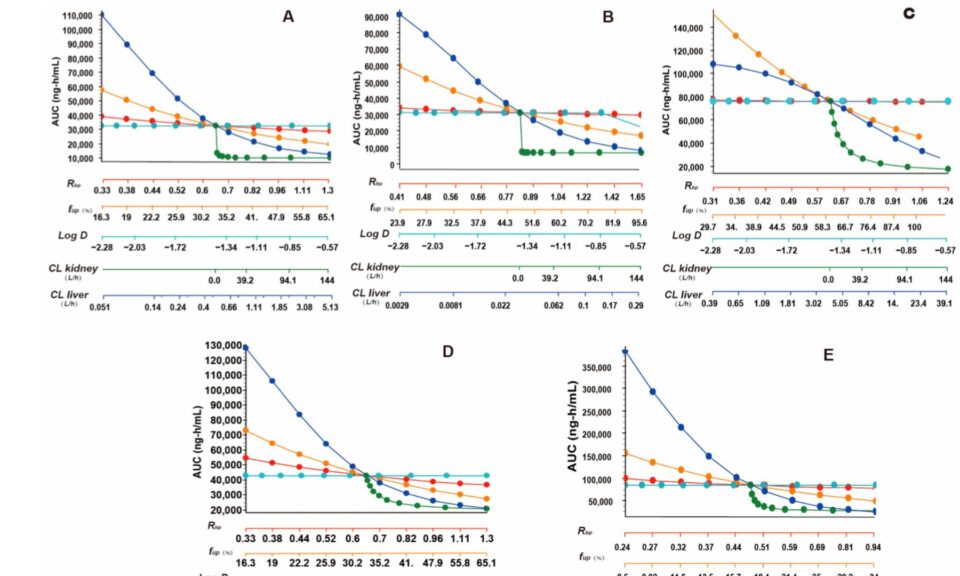
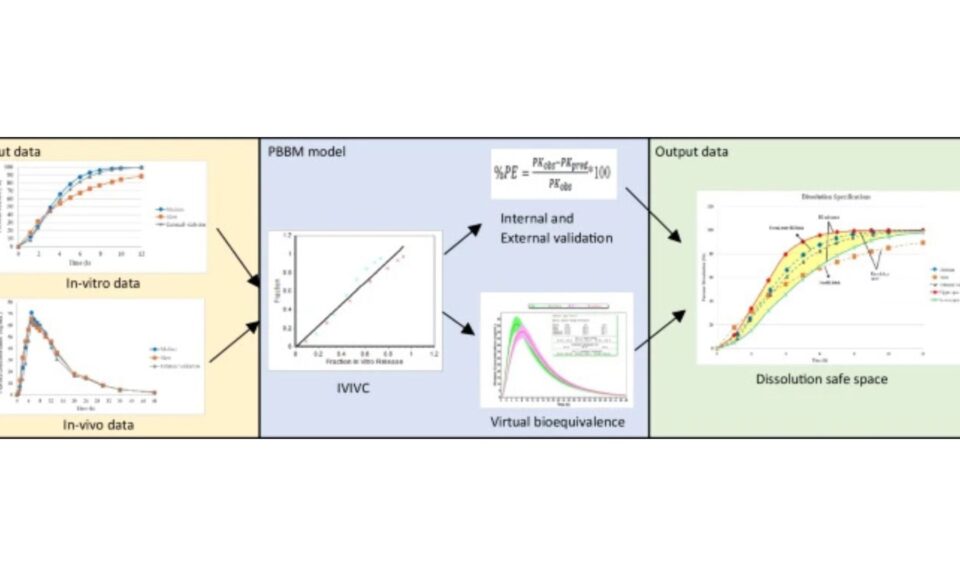
- Deconvoluting in vivo dissolution for mechanistic IVIVCs
- Understanding food effect differences
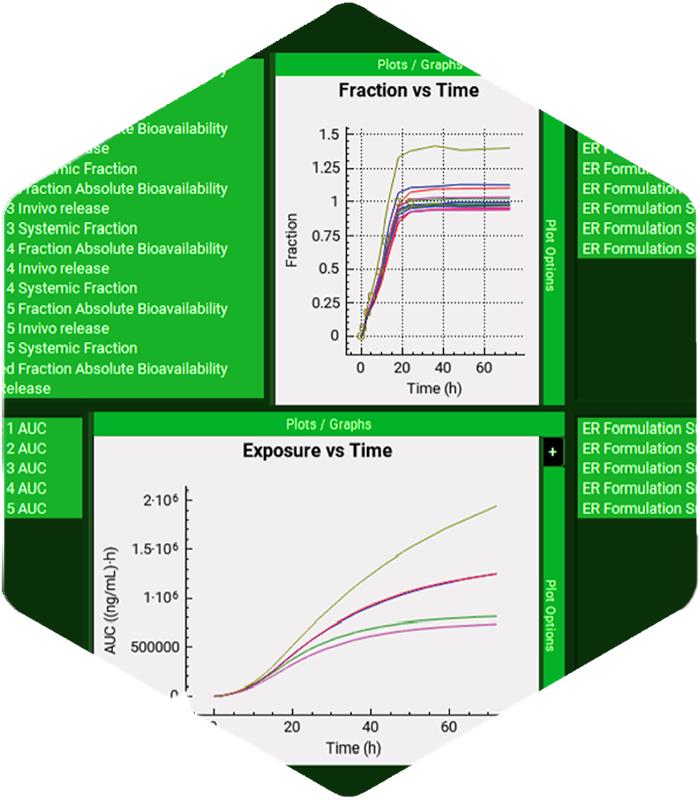
- Conducting virtual population PK & PBPK studies & bioequivalence trials
- Identifying appropriate dose levels and dosing regimens in diseased & pediatric populations
- … and more!
Learn More About Additional Dosage Routes
Read about various case studies by scanning the peer-reviewed publication list! Or, have a project that would benefit from GastroPlus modeling but do not have the expertise in-house to utilize it? Schedule a call with one of our consulting experts today!
- New user-friendly interface
- New refined model-building paradigm
- New views
- Enhanced lab look
- New verification suite
See the GPX release notes for more information.










![Absorption, Distribution, Metabolism, and Excretion of [14C]SHEN211, a Nonpeptidic Small-Molecule 3CLpro Inhibitor, in Rats](https://www.simulations-plus.com/wp-content/uploads/ChatGPT-Image-Jul-15-2025-at-03_34_17-PM-960x576.jpg)