Developing Quantitative Structure-Activity Relationship (QSAR) models applicable to general molecules is of great significance for molecular design in many disciplines.

Accurate Prediction of Liver Fat Reductions Across Range of Weight Loss by Quantitative Systems Pharmacology Modeling
Weight loss has positive effects on reducing hepatic lipid burden in MASH patients. Reports using various...

Formulation development, characterization, and mechanistic PBPK modeling of metoclopramide loaded halloysite nanotube (HNT) based drug-in-adhesive type transdermal drug delivery system
Metoclopramide is an antiemetic agent prescribed for motion sickness, cancer chemotherapy, and pregnancy.

New Features of the IVIVC Module on the GPX Platform
This webinar, in Portuguese, will explore the IVIVC (in vitro–in vivo correlation) module on the innovative GPX platform.

Investigation of the Anti-asthmatic Activity of Solidagenone, In Vitro Toxicity Versus In Silico Studies
Solidagenone, a labdane diterpene isolated from inflorescences of Solidago chilensis Meyen, Asteraceae, was investigated for its anti-inflammatory and anti-asthmatic action.

Ocular Drug Discovery & Development: How Modeling & Simulation is Driving and Optimizing Complex Formulations
The development of ophthalmic drug products is challenging due to the complexity of the ocular system, the lack of sensitive testing to evaluate the interplay of its physiology with ophthalmic drugs, and measurement limitations associated with ocular pharmacokinetics.

Erectile Dysfunction Therapy of Bariatric Patients: Tadalafil Biopharmaceutics and Pharmacokinetics Before vs. After Gastric Sleeve/Bypass
Bariatric surgery introduces significant changes in the gastrointestinal tract, which may affect oral drug absorption/bioavailability.

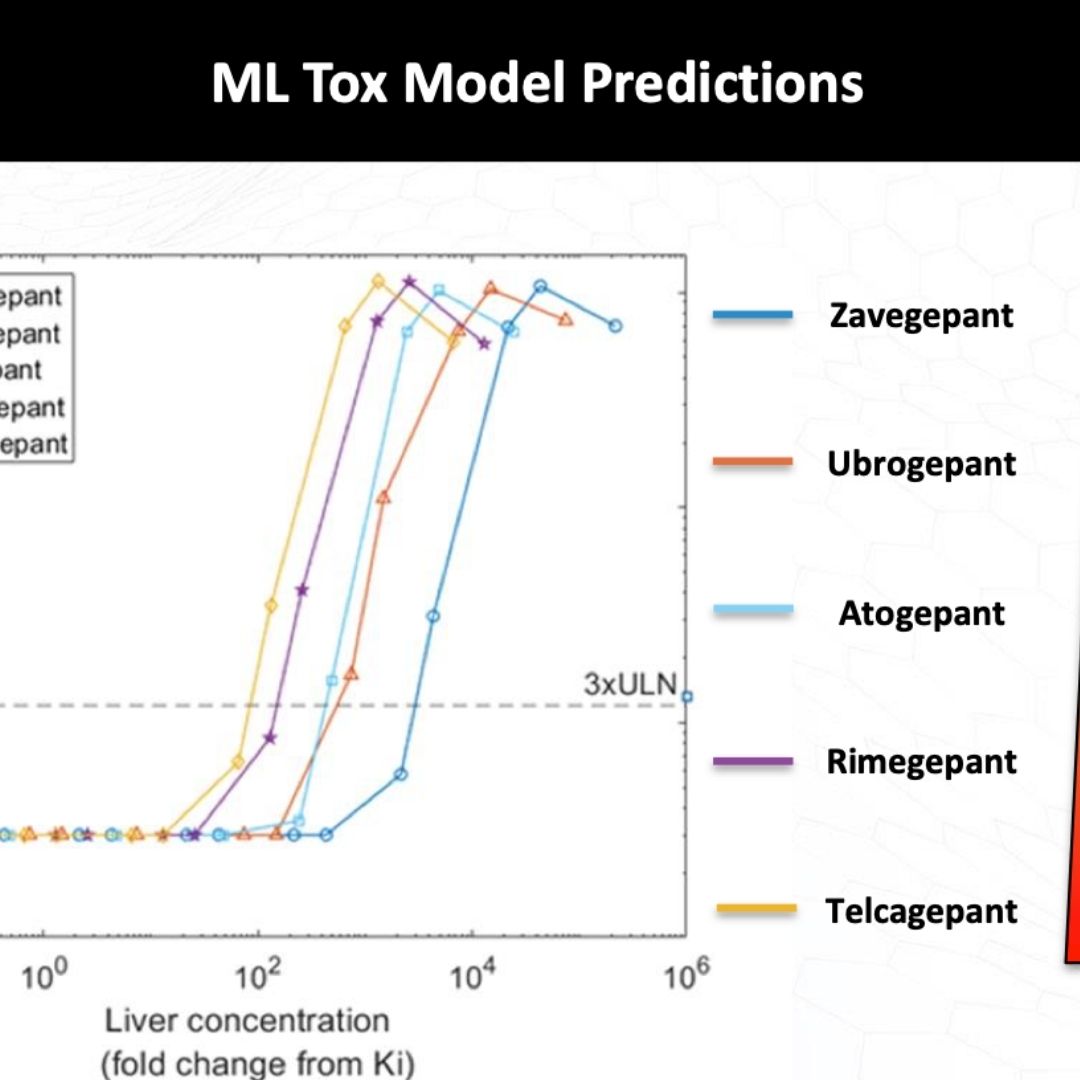
Quantitative Systems Toxicology Modeling of Otenaproxesul Liver Enzyme Elevations Leads to Prediction of Liver Safety for Acute Otenaproxesul Dosing
Otenaproxesul (ATB-346), a drug that combines naproxen with a thiobenzamide antioxidant, is being developed as an NSAID that reduces gut toxicity effects. Liver...

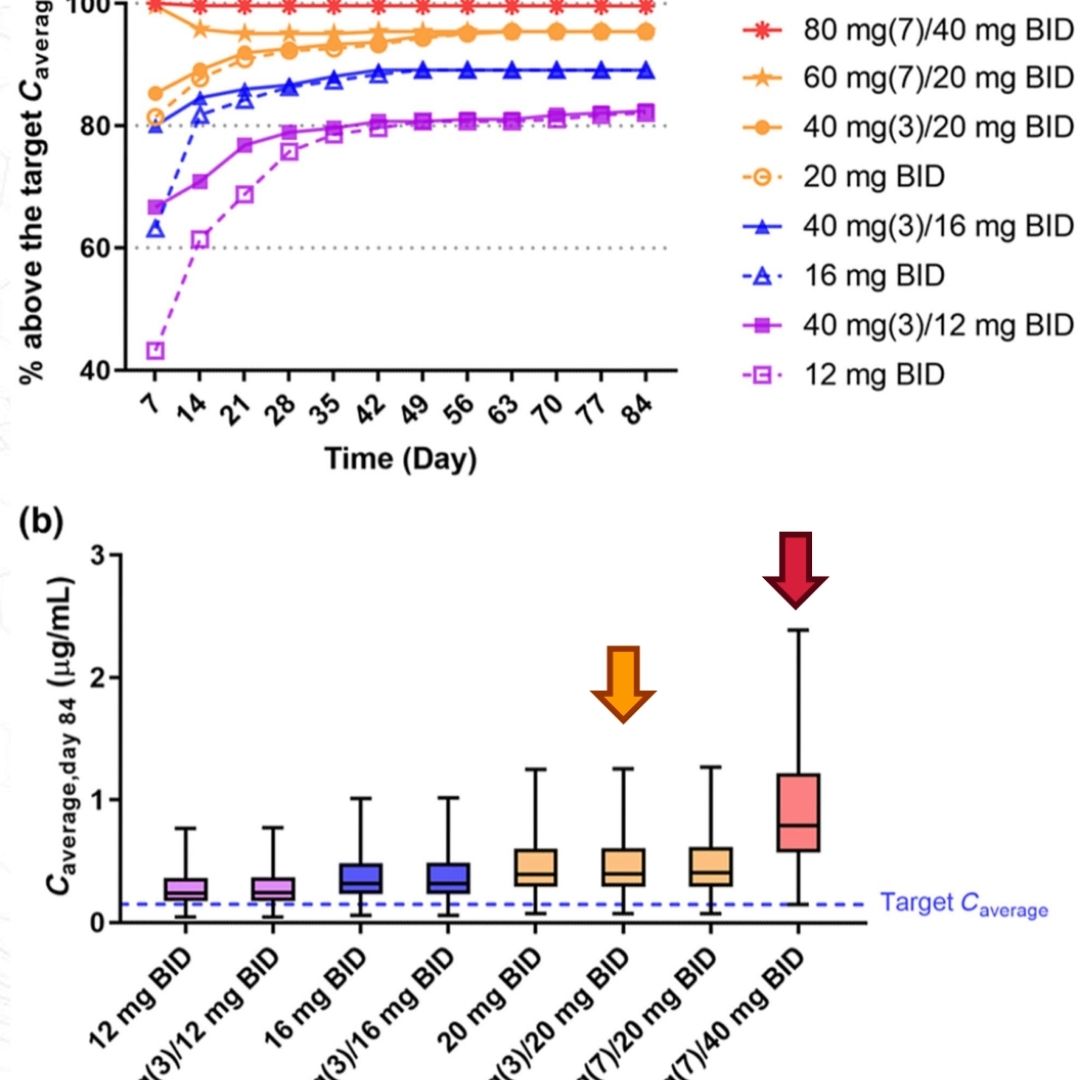
Physiologically Based Pharmacokinetic Models for Infliximab, Ipilimumab, and Nivolumab Developed with GastroPlus® to Predict Hepatic Concentrations
Infliximab (IFX), ipilimumab (IPI), and nivolumab (NIVO) have been associated with hepatotoxicity...

Simulated CD8+ T Cell-Mediated Liver Injury During Ipilimumab Administration in a Simulated Population (SimPops®) Demonstrates Profiles Consistent with Observed Clinical Data
Immune checkpoint inhibitors (ICIs) have revolutionized treatment of various cancers. They act by releasing the brakes on immune responses to permit immune-mediated tumor cell killing...

Weight Loss and Nausea from Subcutaneous and Oral Semaglutide Accurately Simulated with a QSP Model
The recent availability of effective GLP-1R agonist (GLP-1RA) based treatments of obesity has provided great benefit to patients. Understanding the balance between body weight (BW) loss...

Comparison of Sensitivity Analysis Methods in the Context of a QSP Model for Gout
As quantitative systems pharmacology (QSP) models are increasingly used to inform key...

Eculizumab as a Key Comparator for the Evaluation of Complement Targeted Novel Therapeutic Strategies with a QSP Model
Paroxysmal nocturnal hemoglobinuria (PNH) is one of multiple diseases in which complement dysregulation, leading to...

Modeling progression and treatment of prostate cancer using the Thales QSP software platform
Metastatic, castration resistant prostate cancer (mCRPC) is an aggressive form of prostate cancer in...

Simulations Plus and the University of Connecticut Receive New FDA Grant to Expand Mechanistic Modeling Approaches for Long-Acting Injectables
Funded collaboration will leverage current FDA partnership to include additional LAI technologies

Evaluation of Drug–Drug Interactions Between Clarithromycin and Direct Oral Anticoagulants Using Physiologically Based Pharmacokinetic Models
This study assessed the pharmacokinetic (PK) interactions between clarithromycin (a P-glycoprotein [P-gp] inhibitor) and four direct oral anticoagulants (DOACs) (P-gp substrates) using physiologically based PK (PBPK) models to elucidate the influence of P-gp in the interaction between them.

Discovery of Penicillic Acid as a Chemical Probe Against Tau Aggregation in Alzheimer’s Disease
Alzheimer's Disease (AD) is a neurodegenerative disorder proven to be caused by the aggregation of protein tau into fibrils, resulting in neuronal death.