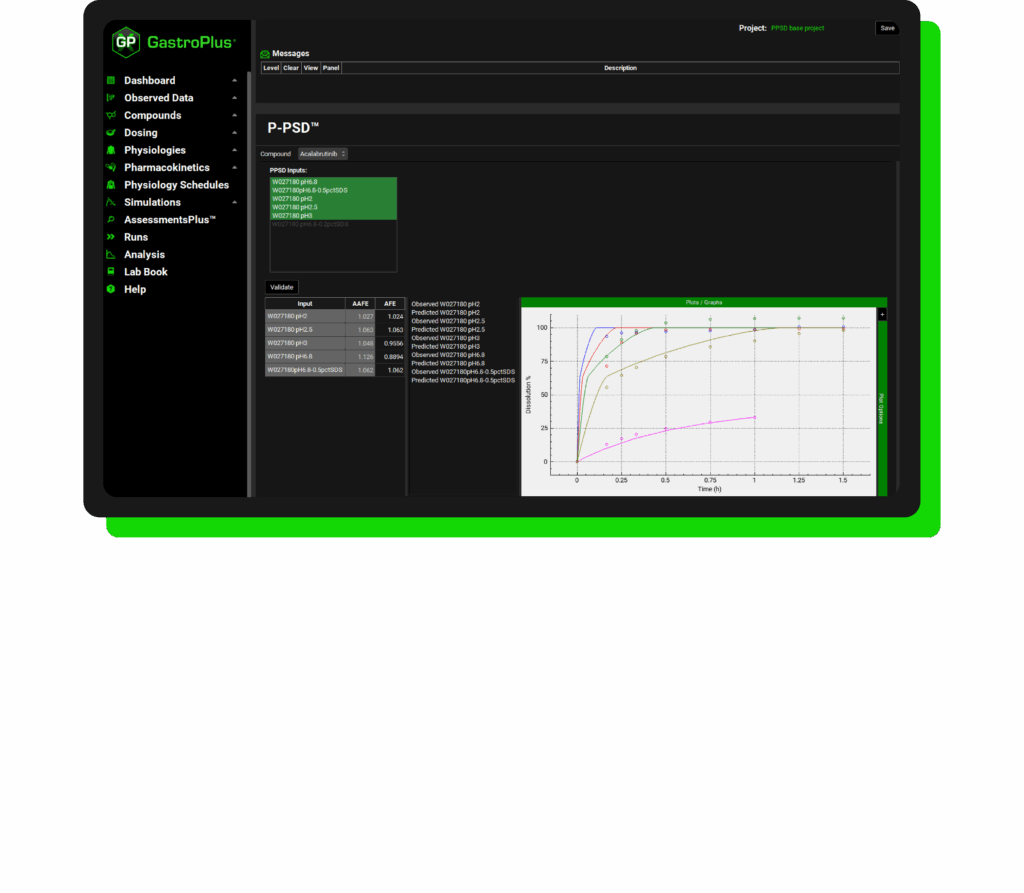
The P-PSD (also known as product-specific particle size distribution) module offers a convenient way to incorporate drug product batch dissolution data into your PBBM models. The P-PSD approach accounts for the effects of excipients, process parameters, and other formulation factors which reflects the product we give to patients. This allows you to incorporate the data you are already collecting and quickly model new formulation strategies confidently.
01. Discover
What is the P-PSD Module?
02. Leverage
Confident Decision-Making Made Easy
The P-PSD module in GastroPlus has been thoughtfully designed by the very scientists and domain experts who pioneered and validated the underlying methodology. This ensures that every aspect of the workflow is grounded in real-world application and scientific rigor, providing users with a solution that is not only intuitive and efficient but also aligned with best practices.
By embedding expert-driven intelligence directly into the interface, we’re gaining greater confidence in model-informed decision making across formulation development, bioequivalence, and regulatory submissions.
03. Resources
Explore Resources

