Drug-induced liver injury (DILI) remains an adverse event of significant concern for drug development and marketed drugs, and the field would benefit from better tools to identify liver liabilities early in development and/or to mitigate potential DILI risk in otherwise promising drugs.

Identification of neuron selective androgen receptor inhibitors
Aim: To identify neuron-selective androgen receptor (AR) signaling inhibitors, which could be useful in the treatment of spinal and bulbar muscular atrophy (SBMA)...
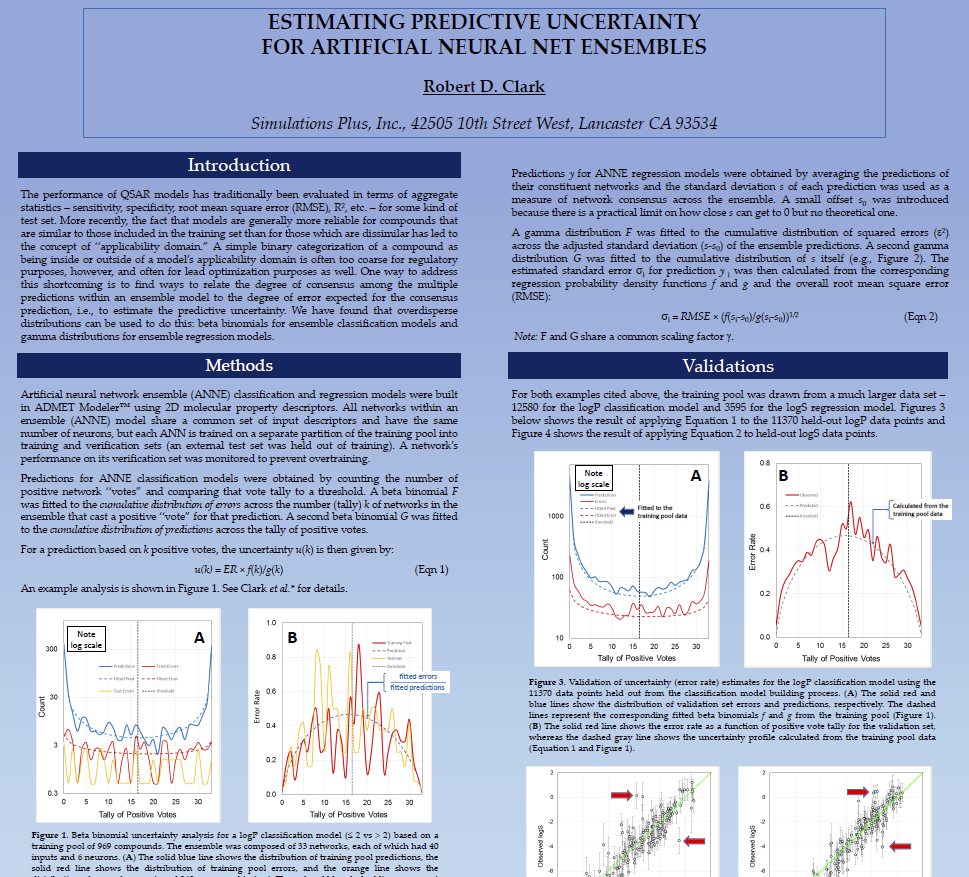
Estimating Predictive Uncertainty for Artificial Neural Net Ensembles
The performance of QSAR models has traditionally been evaluated in terms of aggregate statistics – sensitivity, specificity, root mean square error (RMSE), R2, etc. – for some kind of test set.

Comparison of oral absorption properties among different bisphosphonates by using a newly developed physiologically based pharmacokinetic model.
Absorption of bisphosphonates(BPs)from the gastrointestinal tract is inhibited by forming a complex combined with multivalent cation in intake foods.
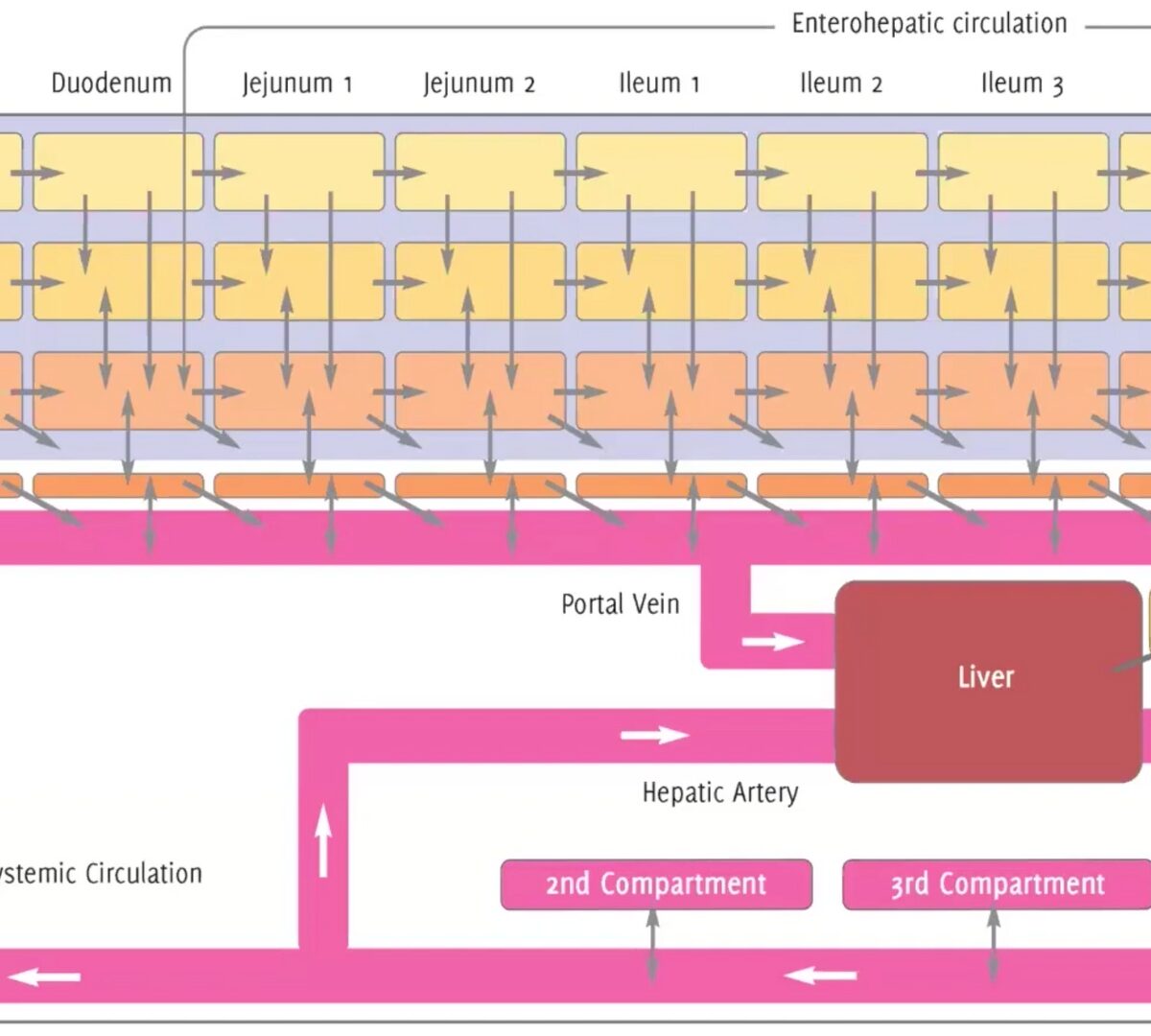
Applying Physiologically-Based Pharmacokinetic (PBPK) Modeling & Simulation to Assist with Pharmaceutical Research and Regulatory Submissions
Open communication between regulatory agencies, pharmaceutical companies, universities, and software providers will help identify new M&S applications.

Comparing Dog and Human Intestinal Fluids: Implications on Solubility and Biopharmaceutical Risk Assessment
Despite many documented differences in gut physiology compared to humans, the beagle dog has been successfully used as a preclinical model for assessing the relative bioavailability of dosage forms during formulation development.

Quantitative analysis of lab-to-lab variability in Caco-2 permeability assays
In this study, Caco-2 permeability results from different laboratories were compared. Six different sets of apparent permeability coefficient (Papp) values reported in the literature were...

Mechanistic investigation of the negative food effect of modified release zolpidem
When administered orally as either an immediate or modified release dosage form, zolpidem demonstrates a negative foodeffect, i.e. decrease in Cmax and AUC.

Exploring Canine – Human Differences in Product Performance. Part II: Use of Modeling and Simulation to Explore the Impact of Formulation on Ciprofloxacin In Vivo Absorption and Dissolution in Dogs
This study explored the in vivo performance of three oral ciprofloxacin formulations (oral solution, fast, or slow dissolving tablets) in beagle dogs.

The impact of supersaturation level for oral absorption of BCS class IIb drugs, dipyridamole and ketoconazole, using in vivo predictive dissolution system: Gastrointestinal Simulator (GIS)
The development of formulations and the assessment of oral drug absorption for Biopharmaceutical Classification System (BCS) class IIb drugs is often a difficult issue due to the potential for...

Dr. Daniel Weiner Joins Simulations Plus Board of Directors
World-renowned scientist, FDA consultant, and experienced manager to reinforce SLP board

Simulations Plus to Acquire DILIsym Services, Inc.
Accretive acquisition to expand Simulations Plus offerings by adding leading provider of drug-induced liver injury (DILI) software and consulting services

Physiologically Based and Population Pharmacokinetic (PBPK, PPK) Modeling and Simulation to Support Dose Selection and Study Design for Eslicarbazepine Acetate (ESL) Adjunctive Therapy in Infants with Partial-Onset Seizures (POS)
Eslicarbazepine acetate (ESL) is a once-daily (QD) oral antiepileptic drug (AED), approved as adjunctive treatment in adults ≥18 years for partial-onset seizures (POS) in the USA and Canada, and as...

Modeling and Simulation Strategy to Support Eslicarbazepine Acetate (ESL) Pediatric Dose Selection in the Treatment of Partial-Onset Seizures (POS) Based on Matching Adult Exposures
Eslicarbazepine acetate (ESL) is a once-daily (QD) oral antiepileptic drug (AED), approved as adjunctive treatment in adults > years for partial-onset seizures (POS) in the USA and Canada, and as monotherapy...

Studies on Core-Shell Nanocapsules of Felodipine: In Vitro-In Vivo Evaluations
The present study aimed for in vitro-in vivo-in silico simulation studies of experimentally designed (32-factorial) Capmul PG-8-cored, Eudragit RSPO-Lutrol F 127 nanocapsules to ferry felodipine using GastroPlus™.

Identification of novel TACE inhibitors compatible with topical application
Targeting the Tumor Necrosis Factor α signalling with antibodies has led to a revolution in the treatment of psoriasis. Locally inhibiting Tumor Necrosis Factor α Converting Enzyme...

Evolution of Circulating Tumor DNA Profile from First-line to Subsequent Therapy in Metastatic Renal Cell Carcinoma
Background: Treatment of metastatic renal cell carcinoma (mRCC) typically entails mechanistically distinct agents across the first- and second-line setting.
