This study aims to design and optimize ondansetron (OND) gastro-retentive floating minitablets for better and prolonged control of postoperative nausea and vomiting (PONV) with improved patient compliance.
The Power of Simulation Training
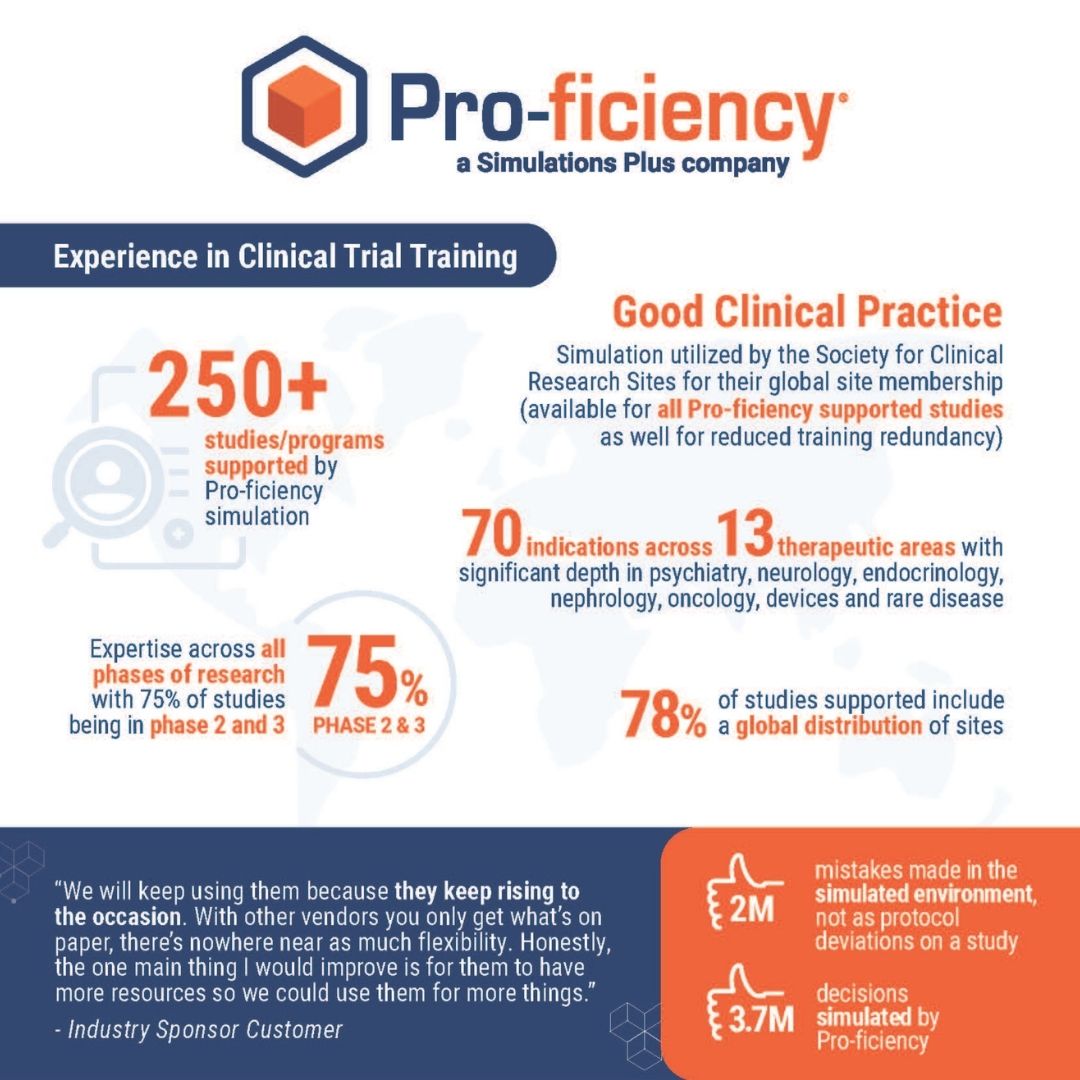
Experience in Clinical Trial Training

Establishing Virtual Bioequivalence and Clinically Relevant Specifications for Omeprazole Enteric-Coated Capsules by Incorporating Dissolution Data in PBPK Modeling
Currently, Biopharmaceutics Classification System (BCS) classes I and III are the only biological exemptions of immediate-release solid oral dosage forms eligible for regulatory approval.

Simulations Plus Announces New Research Project with the International Collaboration on Cosmetics Safety
Objective to define best practices for the use of novel PBK modeling strategies to support animal-free safety assessment of new chemicals.

Key Takeaways on the Acceptability of PBPK from the ICH Harmonised Guideline on Drug Interaction Studies
One of the significant challenges faced by pharmaceutical companies is the sometimes unclear guidance on what data regulators want to see included in their submissions and the format in which it should be provided.

Clinical Ocular Exposure Extrapolation for a Complex Ophthalmic Suspension Using Physiologically Based Pharmacokinetic Modeling and Simulation
The development of generic ophthalmic drug products with complex formulations is challenging due to the complexity of the ocular system and a lack of sensitive testing to evaluate the interplay of its physiology with ophthalmic drugs.

Scientific Content Development
A client was anticipating a new FDA indication for their product that would allow it to be used as a first-line therapy.

Novel Medical Education
A client needed a novel program to re-engage with their key opinion leaders (KOLs) who could serve on a speaker bureau.

Advisory Boards
A client’s cardiopulmonary/respiratory team needed to develop, design, and execute a half-day Regional Advisory Board.

Opinion Leader Management
A client was new to the market and lacked knowledge of their customers and key opinion leaders (KOLs) in the field.

Competitive Intelligence
A global medical affairs client was stretched thin: their internal and field-based medical staff couldn’t be everywhere at once, and they needed help keeping on top of the material presented at key medical conferences.

Market Assessments and Insights
A client had an asset in clinical development that showed activity against multiple tumor types.

Faculty Training & Development
A client needed new patient cases to illustrate recent indication updates to their label. They also wanted to consolidate new and existing case studies into a single, streamlined guide with a new, updated look.

Selective Promotional and Creative
A client’s inflammation medical affairs team wanted to introduce their business unit as an emerging leader with a robust inflammation pipeline at annual global IBD and RA conferences.

NAFLD Enrollment Support
Nonalcoholic Fatty Liver Disease, or NAFLD, studies present unique enrollment challenges that Pro-ficiency directly addresses.

Effect of Hepatic Impairment on Trofinetide Exposures Using an In Silico Physiologically Based Pharmacokinetic Model
Trofinetide is the first drug to be approved for the treatment of Rett syndrome.

Advancing Virtual Bioequivalence for Orally Administered Drug Products: Methodology, Real-World Applications and Future Outlook
Bioequivalence studies are pivotal in generic drug development wherein therapeutic equivalence is provided with an innovator product.

Simulations Plus Reports Third Quarter Fiscal 2024 Financial Results
Total revenue of $18.5 million and diluted earnings per share (EPS) of $0.15
Maintains full-year revenue guidance of $69 to $72 million and updates EPS guidance

The Synthetic Cathinones MDPHP and MDPV: Comparison of the Acute Effects in Mice, in silico ADMET Profiles and Clinical Reports
The 3,4-methylenedioxy-alpha-pyrrolidinohexanophenone (MDPHP) is a synthetic cathinone closely related to 3,4-methylenedioxypyrovalerone (MDPV), one of the most common synthetic cathinones present in the "bath salts".
