Fiscal 2022 revenue increased 16% year-over-year to $53.9 million
Diluted earnings per share increased 28% year-over-year to $0.60
Provides Fiscal 2023 guidance for total revenue of $59.3 million to $62.0 million (+10% to 15%)


Fiscal 2022 revenue increased 16% year-over-year to $53.9 million
Diluted earnings per share increased 28% year-over-year to $0.60
Provides Fiscal 2023 guidance for total revenue of $59.3 million to $62.0 million (+10% to 15%)
![Absorption, distribution, metabolism, and excretion of [14C]Mefuparib (CVL218), a novel PARP1/2 inhibitor, in rats](https://www.simulations-plus.com/wp-content/themes/simulations-plus/library/dist/img/default_square-large.jpg)
Mefuparib (CVL218) is a novel second-generation poly-ADP-ribose polymerase (PARP) inhibitor for cancer treatment.

There has been an increased public interest in developing consumer products containing nonintoxicating cannabinoids, such as cannabidiol (CBD) and cannabigerol (CBG).

Board of Directors announces quarterly dividend of $0.06 per share

Rifampicin (RIF) is an essential part of tuberculosis therapy and the pharmacokinetics (PK) of RIF has been of interest due to its non-linear and auto-induction behavior

Development of generic ophthalmic drugs has been extremely challenging due to the complexity of the ocular system and a lack of sensitive testing tools to evaluate its interplay with ophthalmic formulations.
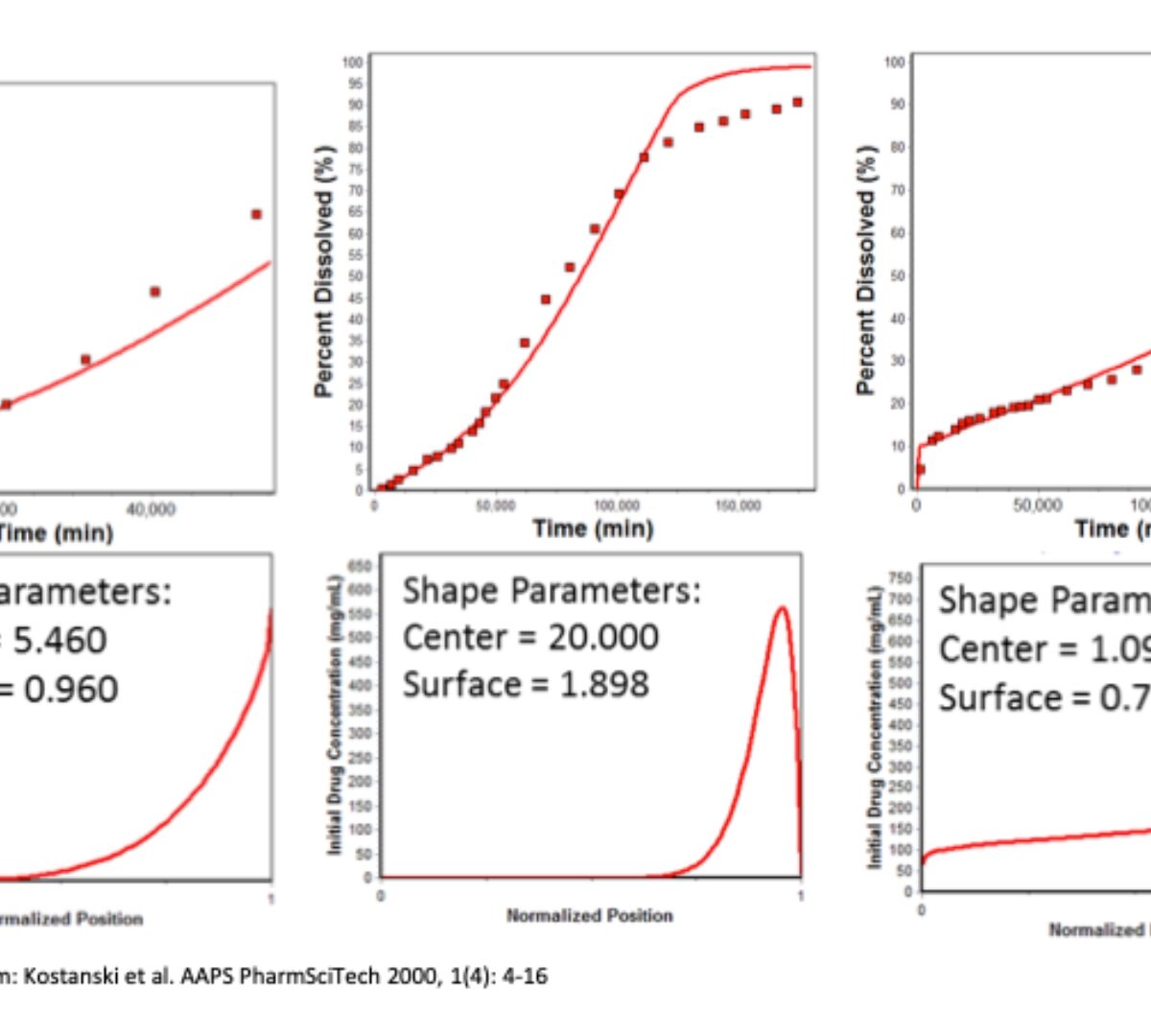
Discuss advances in mechanistic models to simulate in vitro and in vivobehavior of long acting injectables.
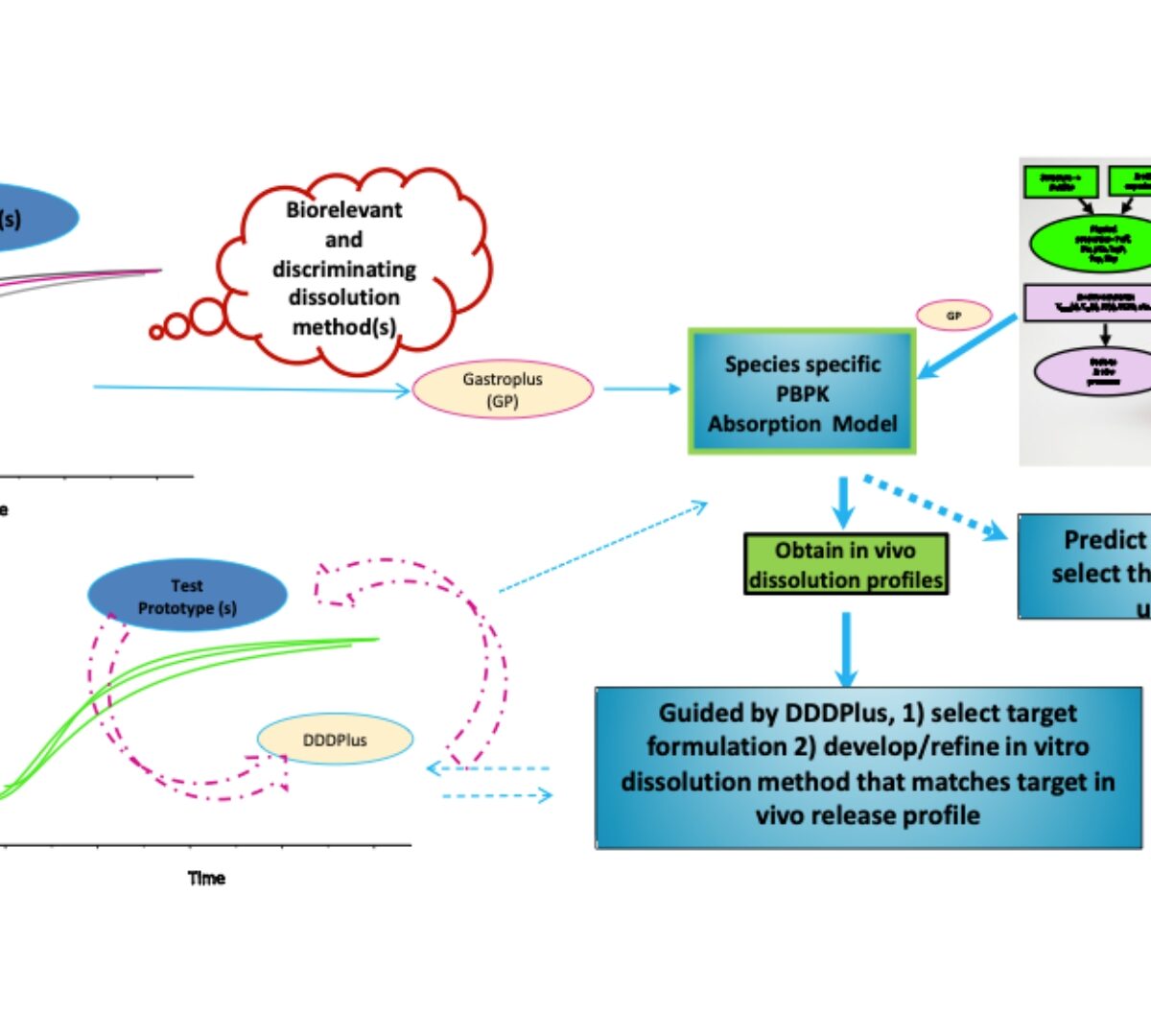
It is challenging to establish a standard approach for moving from preclinical to clinical phases of development. Many hurdles exist during formulation selection and for extrapolation of doses from animals to humans; what may have worked well for one drug candidate may not be appropriate for others. This presentation will provide a general framework/strategy to follow when moving from preclinical to clinical studies (FIH) based on risk identification and mitigation focusing on the application of mechanistic modeling and simulation (PBPK).
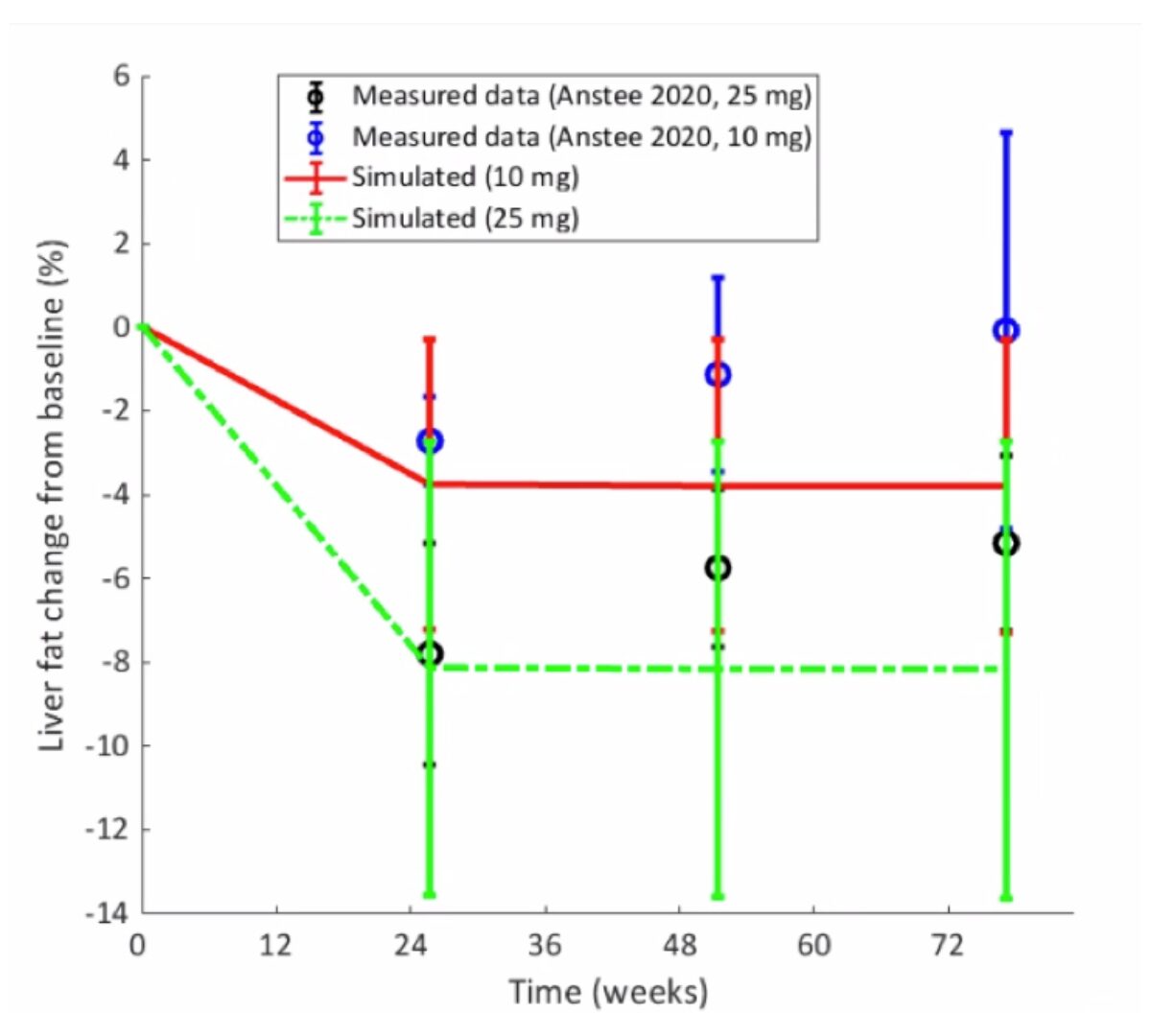
Obeticholicacid (OCA), a bile acid analog and agonist of the farnesoidX receptor (FXR), is currently in clinical trials for the treatment of non-alcoholic steatohepatitis (NASH).

Long acting injectable (LAI) formulations administered through subcutaneous (SC) or intramuscular (IM) routes provide sustained drug release over an extended period.
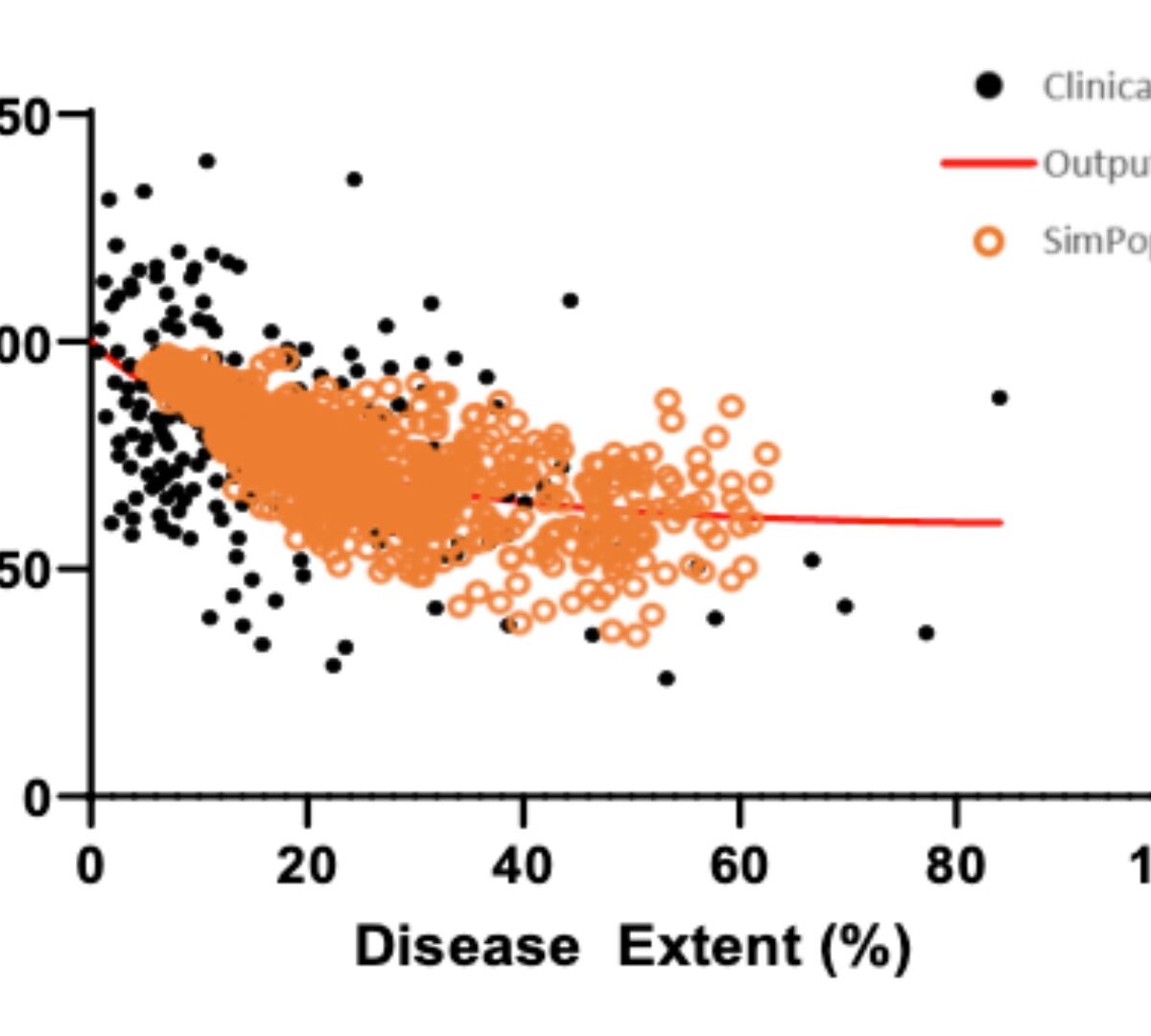
Systemic sclerosis (SSc) is a rare connective tissue and autoimmune disease associated with inflammation of the skin and internal organs.

BAY1128688, a selective inhibitor of aldo-keto reductase family 1 member C3 (AKR1C3), was in clinical trials as a potential therapy to provide pain relief for women with endometriosis.

Breaking News! The new version of GP (version 9.8.3) is now available for download.

New update expands the library of virtual populations and enhances connections between software platforms

Prediction of rat, dog, monkey, and human volume of distribution (VDss) by Rodgers-Lukacova model was evaluated using a data set of more than 100 compounds.

In relation to the registration of generic products, waivers of in vivo bioequivalence studies (biowaivers) are considered in three main cases: certain dosage forms for which...

The safety and efficacy of a generic product are partly based on demonstrating bioequivalence to the innovator product; however, when the innovator product is no longer available as a comparator product...