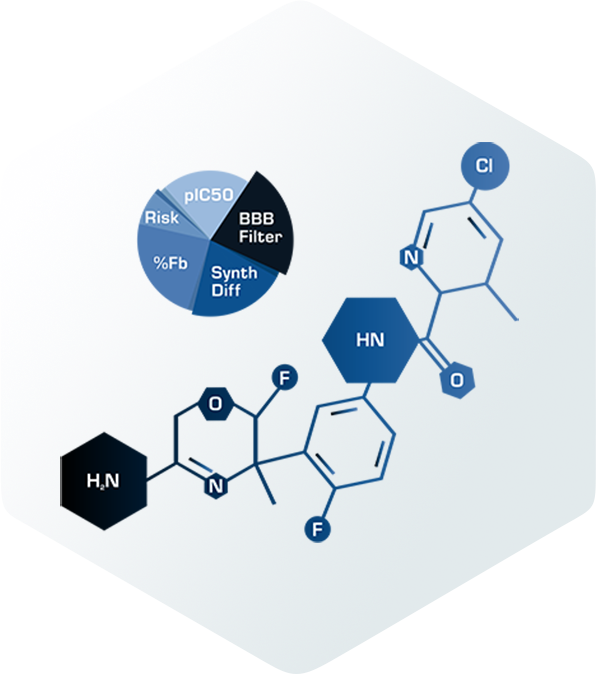
In drug development, uncertainty is inevitable—but costly surprises don’t have to be.
We help teams like yours replace trial-and-error with foresight. Our AI-assisted modeling and simulation platforms integrate mechanistic science, data-driven intelligence, and scalable cloud compute to predict efficacy, safety, dosing, and clinical performance earlier in development. The result is faster learning, smarter investment, and a clearer return on R&D spend – and supporting a new approach methodology (NAM) future.