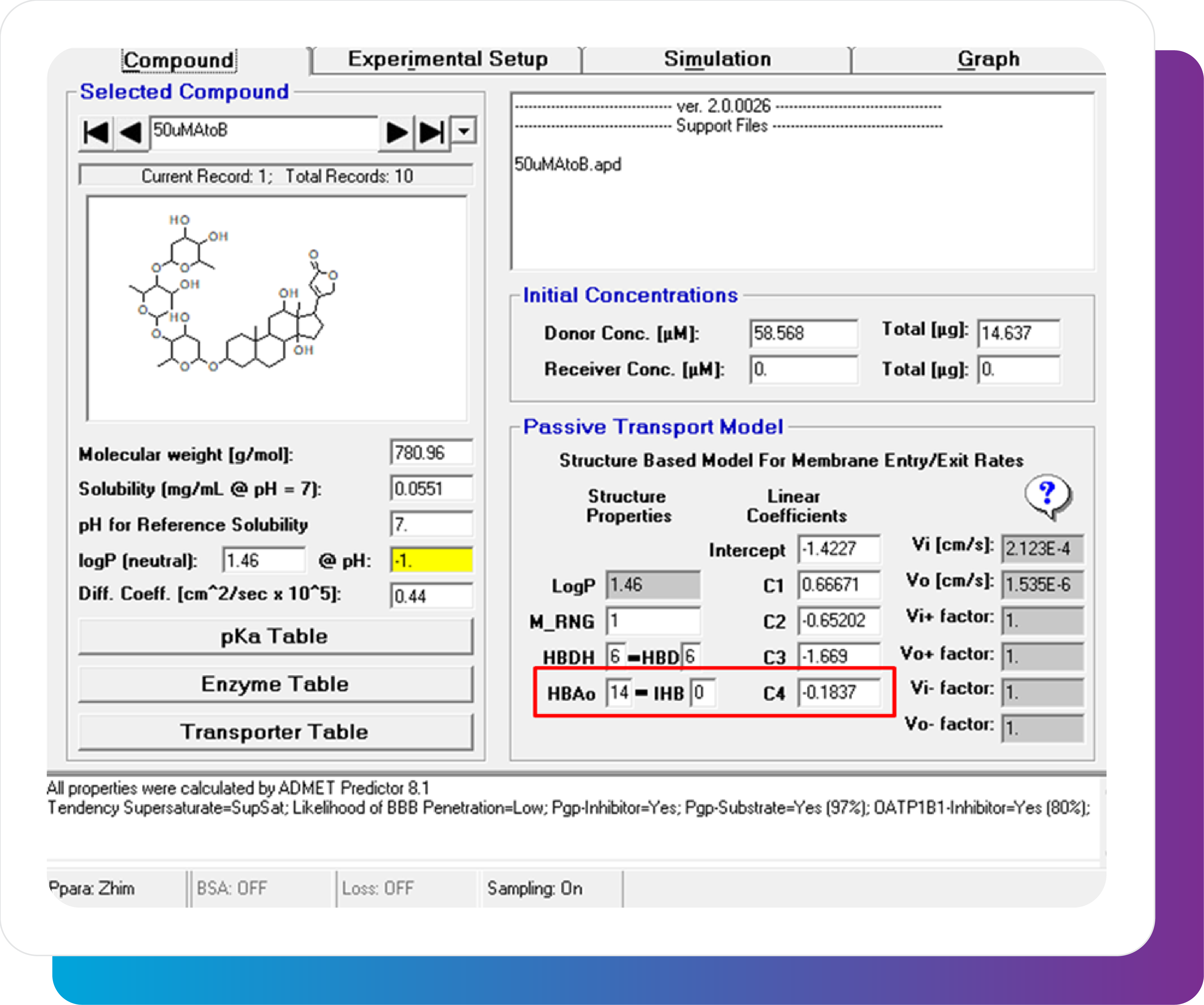
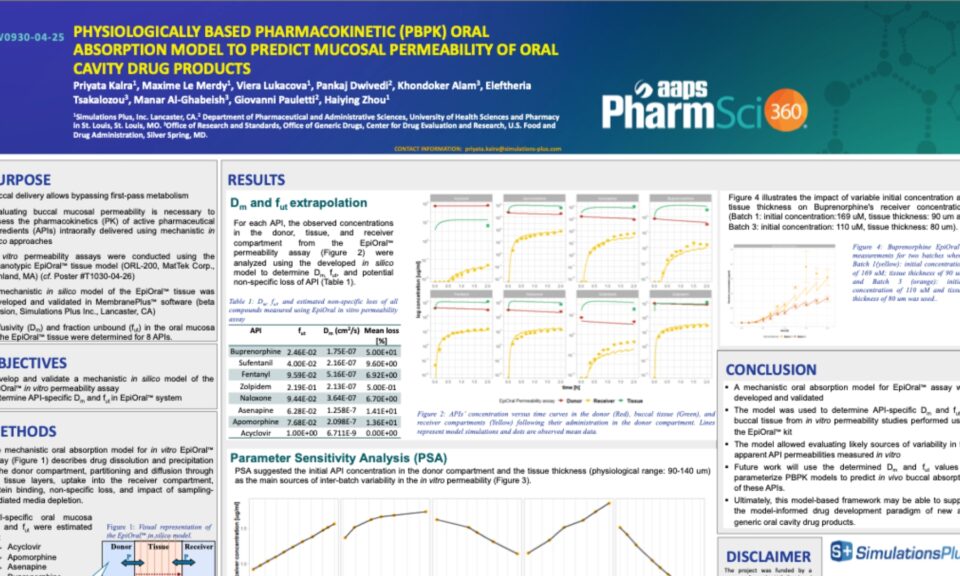
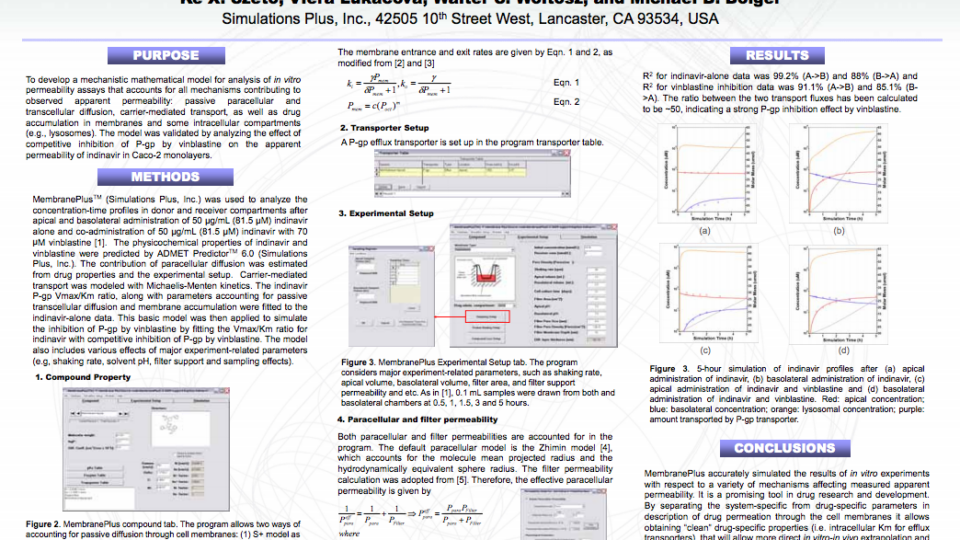
The MembranePlus permeability modeling platform has been utilized by companies across various industries and departments since 2014. MembranePlus has several primary applications:
Simulation of in vitro permeability & hepatocyte concentrations
- Screen compound libraries and prioritize compounds for testing
- Predict various permeability (e.g., paracellular, transcellular) & hepatocyte (e.g., diffusional clearance, lysosomal trapping, biliary excretion) processes
- Estimate different intracellular concentrations, including:
- Membrane
- Cytosol
- Lysosome
- Assess impact of experimental variability on the predicted outcomes
Analysis of measured in vitro experimental data
- Identify paracellular vs. transcellular permeability values
- Calculate in vitro Km and Vmax for enzymes and transporters
- Determine the impact of lysosomal trapping
- Assess biliary excretion routes with sandwich hepatocyte assays
- Fit parameters to build a robust model and unlock important insight from the data
The program provides several simulation modes to help you achieve your goals:
-
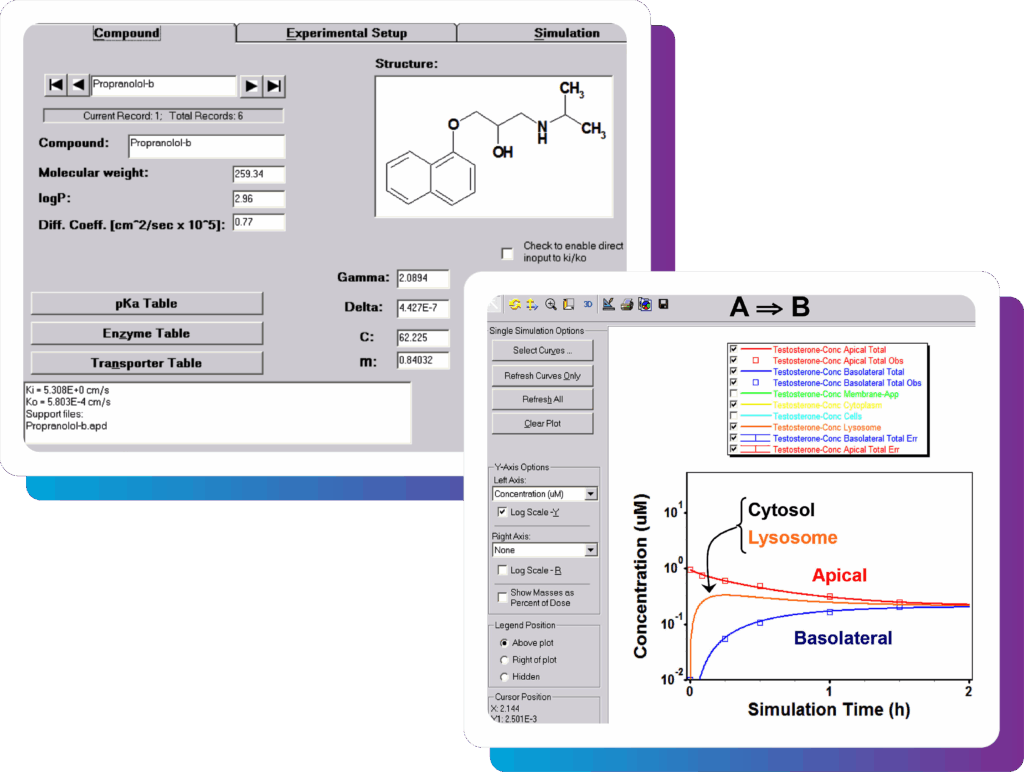
Single simulation – based on your drug properties (whether measured or predicted through ADMET Predictor) and permeability or hepatocyte experimental setup, easily run a simulation to predict the time course changes in concentrations (e.g., apical, basolateral, cellular or lysosomal).
-
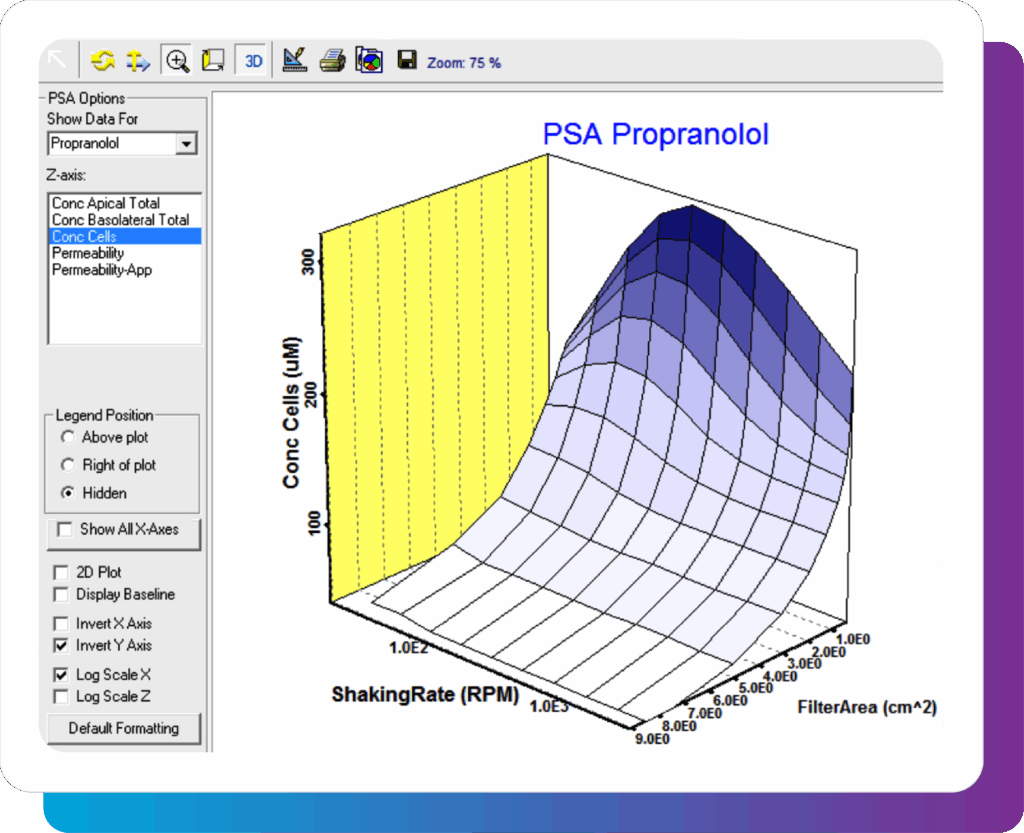
Parameter sensitivity analysis (PSA) – during early drug development, researchers have a large number of compounds to evaluate and limited resources. With Parameter Sensitivity Analysis, quickly assess the impact of changes to certain properties (e.g., physicochemical or experimental) on critical endpoints. This can help guide your resource allocation plans and identify which permeability or hepatocyte experiments should be done next.
-
Batch mode – quickly screen a library of compounds based on predicted permeability or run the same compound through a series of different Caco-2, PAMPA, MDCK, or hepatocyte experiments. Easily prioritize compounds in any way you like: from high to low permeability, based upon potential issues with lysosomal trapping, etc.
Check out various case studies using MembranePlus and GastroPlus® by reviewing publications and webinars on our Resource Center.