DILIsym is Quantitative Systems Toxicology (QST) software capable of predicting and explaining Drug-Induced Liver Injury (DILI).

GastroPlus®X (GPX™) Product Brochure
GastroPlus X (GPX™) is a mechanistically based simulation software package that simulates intravenous and oral absorption, pharmacokinetics, drug-drug interactions and pharmacodynamics in humans and animals.

ADMET Predictor® 11 Product Brochure
Proven algorithms. Premium data. Predictions you can rely on.

AI-Driven Drug Design (AIDD) Flyer
Drug Design, Meet PBPK

Early Drug Discovery (EDD) Flyer
Unleash the Power of AI for Early Drug Discovery
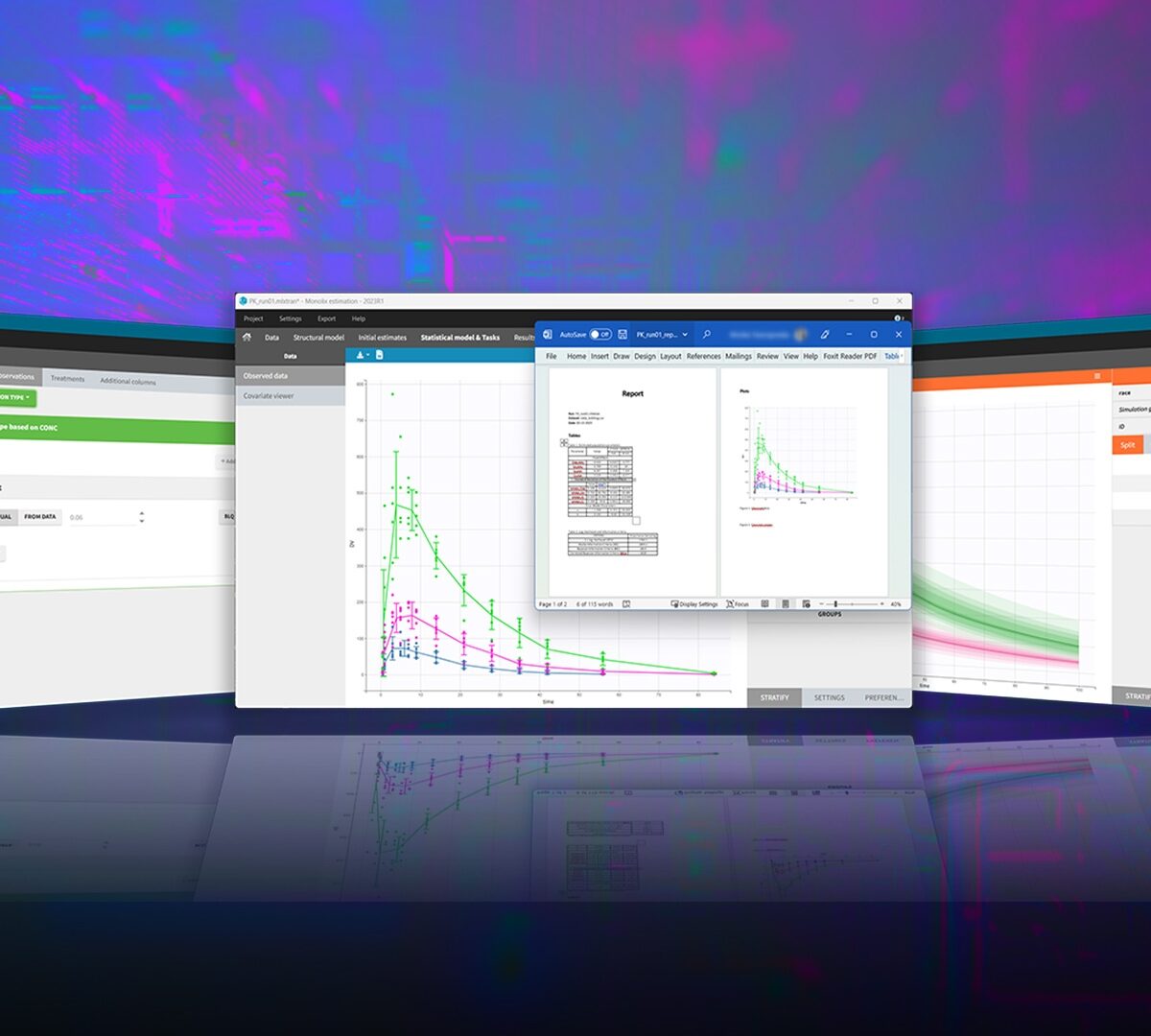
MonolixSuite™ v2023 Product Brochure
A fast easy-to-use and powerful suite of applications for pharmacometrics analysis, modeling and simulation.

DDDPlus™ Product Brochure
Simulations software for the in vitro dissolution experiment of pharmaceutical dosage forms

NAFLDsym® 2B Product Brochure
NAFLDsym® is Quantitative Systems Pharmacology (QSP) Software Capable of Exploring and Predicting Efficacy for Novel NASH Treatments.

Plug n’ Play PBBM
The GastroPlus Plug n' Play PBBM Packages

GastroPlus® Additional Dosage Routes Brochure
Trust Our Gut... and Model Everywhere!

MembranePlus v3 Product Brochure
Your modeling tool for IVIVE of absorption and systemic clearance/distribution processes…

IPFsym™ Product Brochure
State of the art QSP software for idiopathic pulmonary fibrosis (IPF)
