Acute kidney injury (AKI) is often initiated by cellular toxicity of proximal tubule epithelial cells (PTEC) when...

Furthering the clinical development of navicixizumab in advanced epithelial ovarian cancer patients with a population pharmacokinetic model and exploratory exposure-safety-efficacy response analyses
Navicixizumab is a first-in-class, bispecific, anti-angiogenic antibody to vascular endothelial growth factor (VEGF) and

Using artificial intelligence to design BACE1 inhibitors
β-secretase 1 (BACE1) is an enzyme involved in production of amyloid-βpeptides, which are involved in the pathology of Alzheimer’s disease.
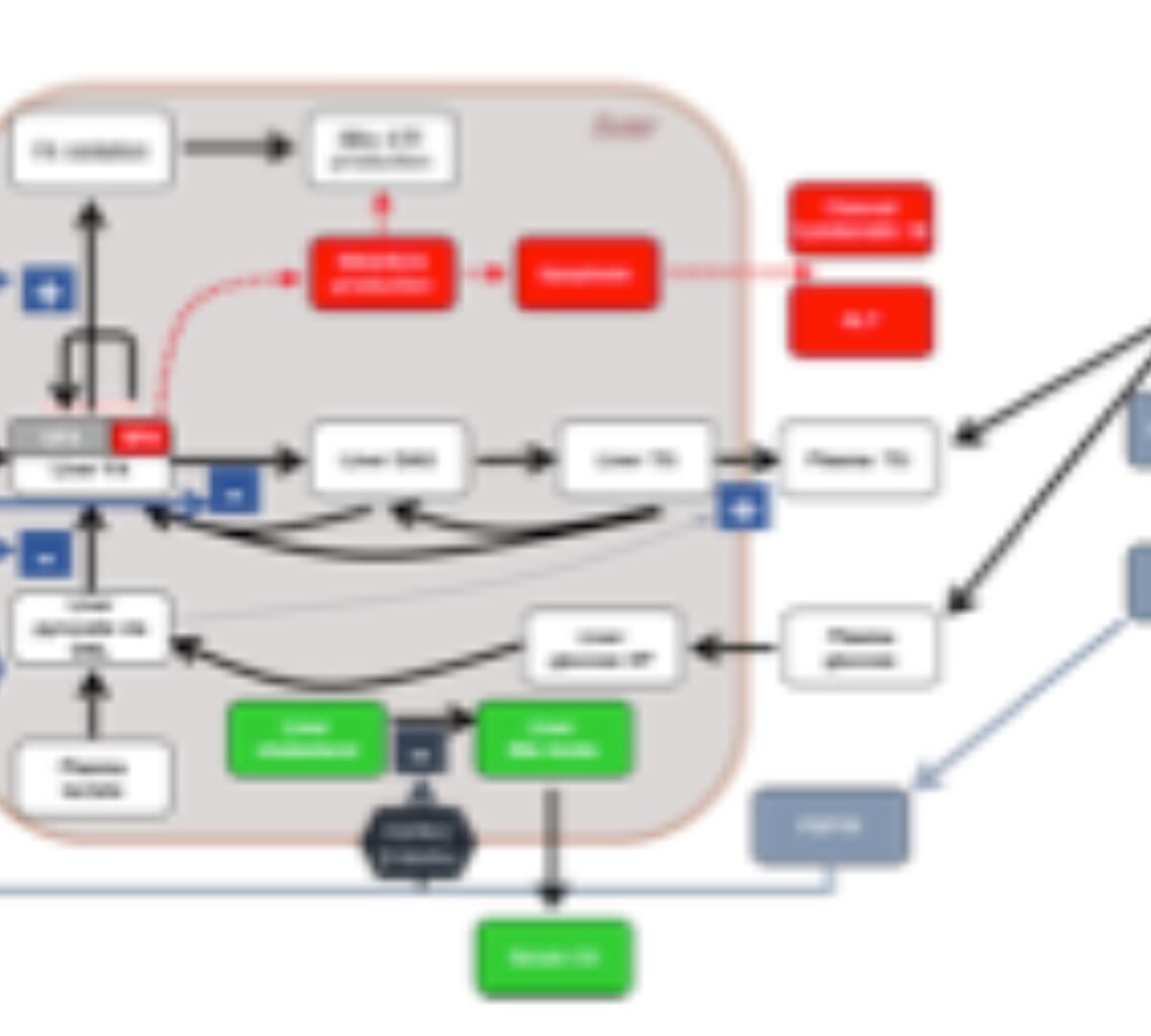
Quantitative Systems Pharmacology Modeling of FGF19 Pathway Using NAFLDsym Prospectively Predicted Liver Fat and Serum Biomarker Responses to MET409 in NASH Patients
Treatment of nonalcoholic steatohepatitis (NASH) is a significant unmet medical need. In this work...

Proof-of-concept Simulations Using BIOLOGXsym, a Novel Quantitative Systems Toxicology (QST) Modeling Platform for Predicting Biologics-induced Liver Injury (BILI), Recapitulate Clinically Observed Hepatotoxicity of GGF2
While biologics continue to address various unmet medical needs, BILI can terminate clinical development...

Population pharmacokinetic modeling and stochastic simulations to support pediatric dose selection of pimavanserin
pimavanserin-is-a-selective-serotonin-receptor-modulating-agent-with-inverse-agonist-antagonist-activity-at-the-5ht2a-receptor-and-to-a-lesser

Validation of non-compartmental analysis (NCA) and bioequivalence results of PKanalix with respect to Phoenix WinNonLin
Non-compartmental analysis (NCA) is used at all stages of drug development and is a key method to understand the pharmacokinetic properties of a compound.

Quantitative Systems Pharmacology (QSP) Model Predicts Lack of Efficacy for Cenicriviroc, a CCR2/5 Antagonist, in NAFLD/NASH Patients
A phase 3 clinical trial (AURORA) for cenicriviroc (CVC), a CC chemokine receptor 2 and 5 (CCR2/5) antagonist, was recently...

Advancing Calcitonin Gene-Related Peptide Receptor Antagonists Using Quantitative Systems Toxicology Modeling to Characterize Next-in-Class Compounds Compared to the Hepatotoxic First in Class Telcagepant
While CGRP receptor antagonists have demonstrated efficacy in the acute and preventive treatment of migraine...

Yo-Yo Dieting Predicted to Contribute to Fibrosis Score Reductions in Untreated (Placebo) Cohorts
10-25% of the patients in placebo cohorts in NASH clinical trials (0.5-2 years) have fibrosis score reductions.

Suppressing TGF-β activation to reduce lung fibrosis: In search for an efficacious dose regimen for alpha V integrin inhibitors
Elevated levels of TGF-β and specifically its activated form is regarded as one of the disease drivers in patients with idiopathic pulmonary fibrosis (IPF).

A library of parent –metabolite models for the MonolixSuite
Ready to use large combination of models integrated in MonolixSuite 2021 with modular filters -a simple workflow to help you test many different hypothesis.

Population Pharmacokinetics Analysis for Molnupiravir in Adults with COVID-19
There is an urgent need for safe and effective oral treatments for COVID-19 that reduce progression to severe illness and risk of hospitalization or death

Optimizing the sample size of a bridging study using Simulx, an application of the MonolixSuite
Plan a bridging study for approval in China

CARDIOsym QSP model prediction of wound healing response following myocardial infarction
Objectives: Cardiac wound healing post-myocardial infarction represents a complex balance between inflammation and fibrosis (Richardson 2017). A biphasic...

Quantitative Systems Toxicology (QST) Modeling of Drug-Induced Acute Proximal Tubule Epithelial Cell Injury and Associated Renal Hemodynamic Responses
Renal proximal tubule epithelial cells (RPTEC) are vulnerable to drug-induced toxicities which often result in acute kidney...

Mechanistic Modeling of Kidney-Injury Molecule 1 (KIM-1) as a biomarker for Cisplatin-Induced Acute Kidney Injury
Kidney Injury Molecule 1 (KIM-1) is a specific and sensitive biomarker for drug-induced kidney injury (AKI) prediction...

Clinical Ocular Exposure Extrapolation Using PBPK Modeling and Simulation: Levofloxacin Solution Case Study
Development of generic ophthalmic drug products is challenging due to the complexity of the ocular system and a lack of sensitive testing tools to evaluate its interplay with ophthalmic formulations

Assessing the Potential for Hepatotoxicity for Combination Therapy of Valproate (VPA) and Cannabidiol (CBD) using Quantitative Systems Toxicology (QST)
Highly purified cannabidiol (CBD) (approved as Epidiolex in the US) is efficacious in treating seizures associated with Dravet syndrome (DS), Lennox-Gastaut syndrome (LGS), and Tuberous Sclerosis Complex (TSC).

Quantitative Systems Pharmacology Modeling Using NAFLDsym Recapitulated Clinically Observed Histological Responses and Serum Markers to NGM282 in NASH Patients
Nonalcoholic steatohepatitis (NASH) is a growing clinical concern, but currently there is no approved medicine for treatment of NASH. Fibroblast growth...
