Biologics address a range of unmet medical needs. However, there are
increasing numbers of BILI cases which slow therapeutic development...


Biologics address a range of unmet medical needs. However, there are
increasing numbers of BILI cases which slow therapeutic development...

Inhibition of efflux transporters located on the canalicular membrane of hepatocytes is an important mechanism of cholestatic liver injury, which is...

Biologics continue to address various unmet medical needs, but the occurrence of BILI can terminate clinical...

Indinavir, a protease inhibitor used to treat patients with HIV/AIDS, is known to induce crystal nephropathy. This is...

Acute kidney injury (AKI) is often initiated by cellular toxicity of proximal tubule epithelial cells (PTEC) when...

Navicixizumab is a first-in-class, bispecific, anti-angiogenic antibody to vascular endothelial growth factor (VEGF) and

β-secretase 1 (BACE1) is an enzyme involved in production of amyloid-βpeptides, which are involved in the pathology of Alzheimer’s disease.
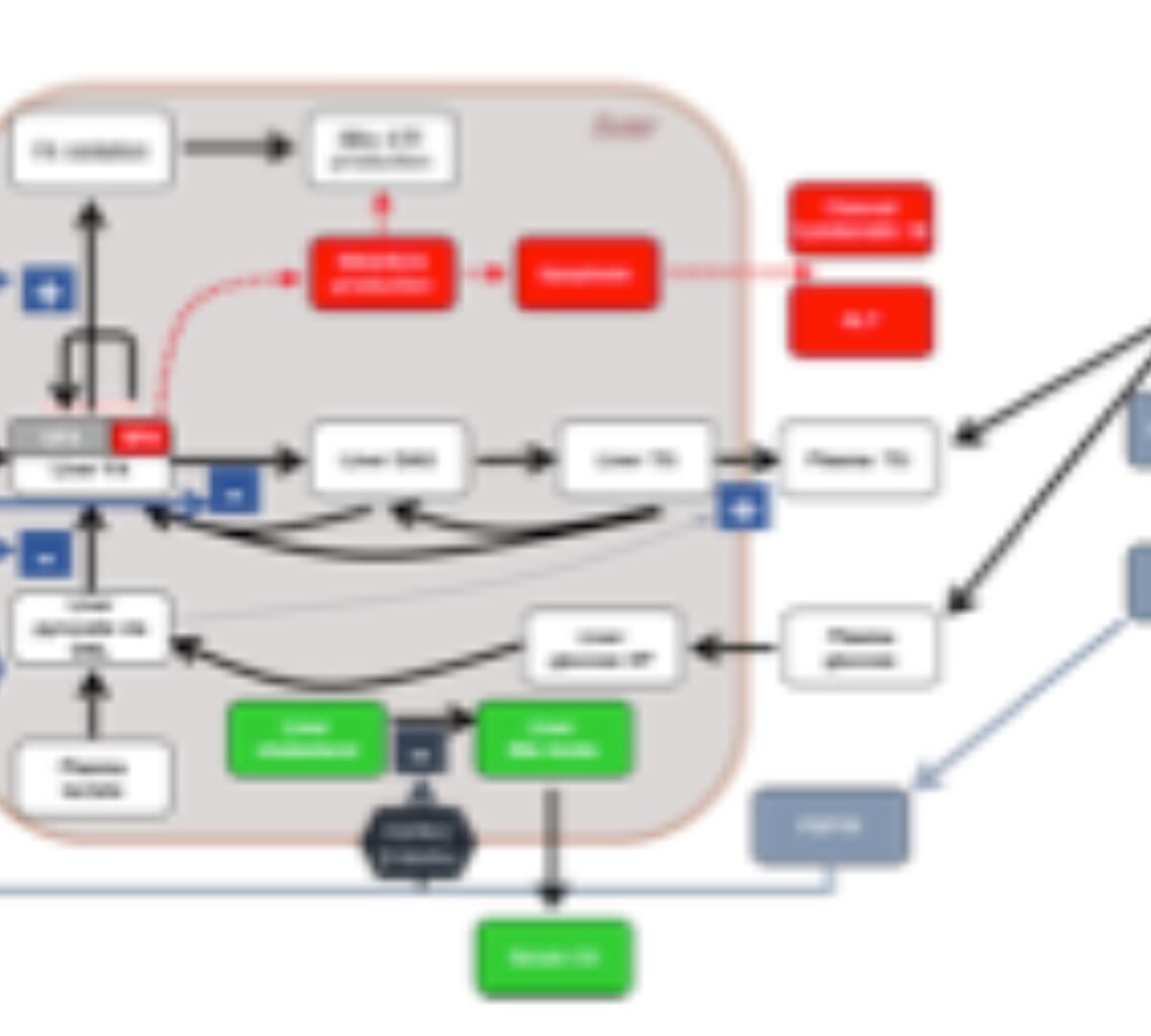
Treatment of nonalcoholic steatohepatitis (NASH) is a significant unmet medical need. In this work...

While biologics continue to address various unmet medical needs, BILI can terminate clinical development...

pimavanserin-is-a-selective-serotonin-receptor-modulating-agent-with-inverse-agonist-antagonist-activity-at-the-5ht2a-receptor-and-to-a-lesser

Non-compartmental analysis (NCA) is used at all stages of drug development and is a key method to understand the pharmacokinetic properties of a compound.

A phase 3 clinical trial (AURORA) for cenicriviroc (CVC), a CC chemokine receptor 2 and 5 (CCR2/5) antagonist, was recently...

While CGRP receptor antagonists have demonstrated efficacy in the acute and preventive treatment of migraine...

10-25% of the patients in placebo cohorts in NASH clinical trials (0.5-2 years) have fibrosis score reductions.

Elevated levels of TGF-β and specifically its activated form is regarded as one of the disease drivers in patients with idiopathic pulmonary fibrosis (IPF).

Ready to use large combination of models integrated in MonolixSuite 2021 with modular filters -a simple workflow to help you test many different hypothesis.

There is an urgent need for safe and effective oral treatments for COVID-19 that reduce progression to severe illness and risk of hospitalization or death

Plan a bridging study for approval in China

Objectives: Cardiac wound healing post-myocardial infarction represents a complex balance between inflammation and fibrosis (Richardson 2017). A biphasic...

Renal proximal tubule epithelial cells (RPTEC) are vulnerable to drug-induced toxicities which often result in acute kidney...