Physiologically based pharmacokinetic (PBPK) modeling is a core element of the early drug and formulation development workflow, enabling data-driven decisions that accelerate timelines and de-risk programs.

GastroPlus® X.2: The Deep Dive Webinar Series – How New AI-Powered Tools Can Support Your PBPK Modeling
What if you could design a complex workflow, then set it into motion with a single click?
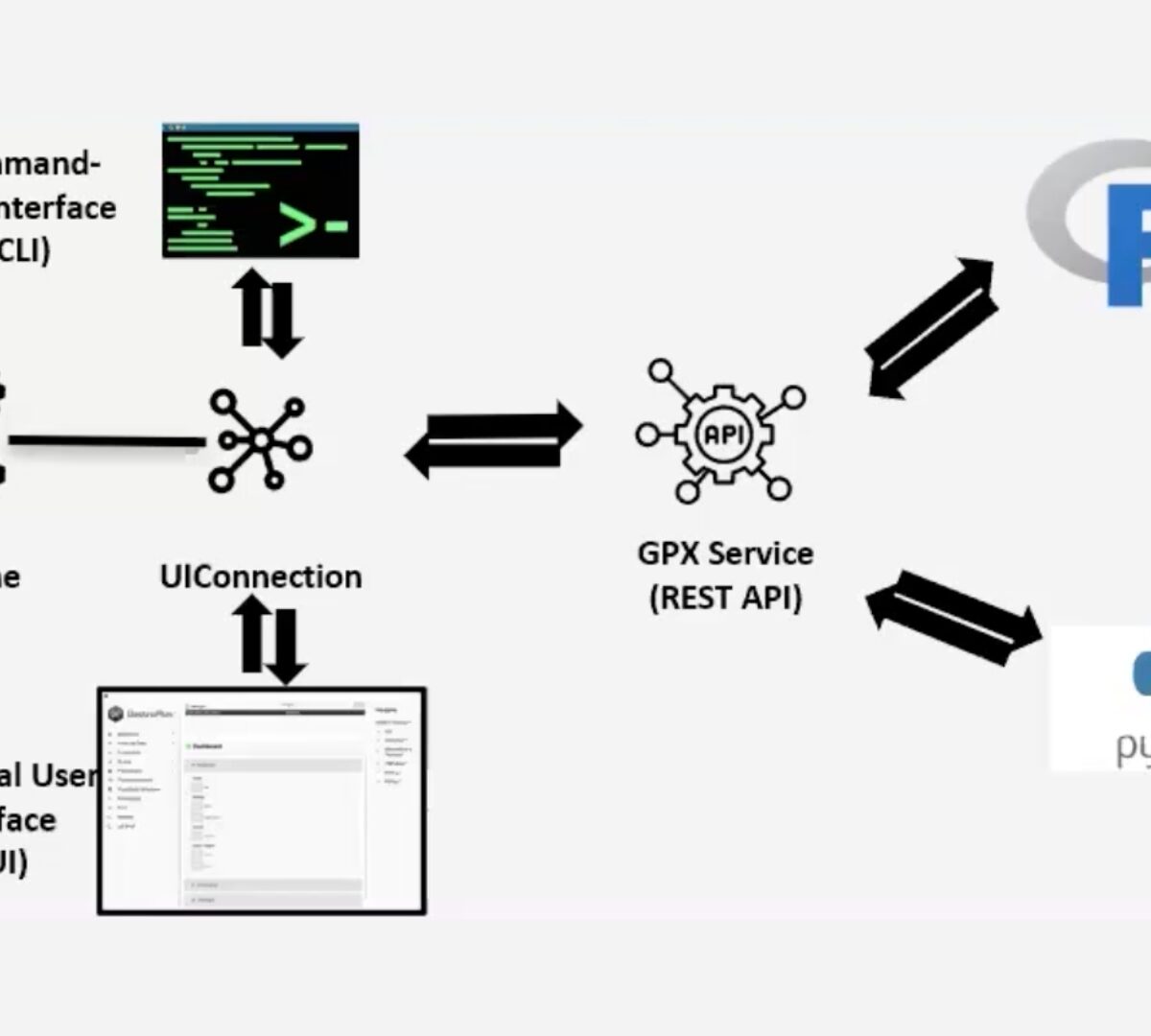
GastroPlus® X.2: The Deep Dive Webinar Series – Introducing Orchestrator, Automation for Complex PBPK/PBBM Modeling in GPX.2
What if you could design a complex workflow, then set it into motion with a single click?
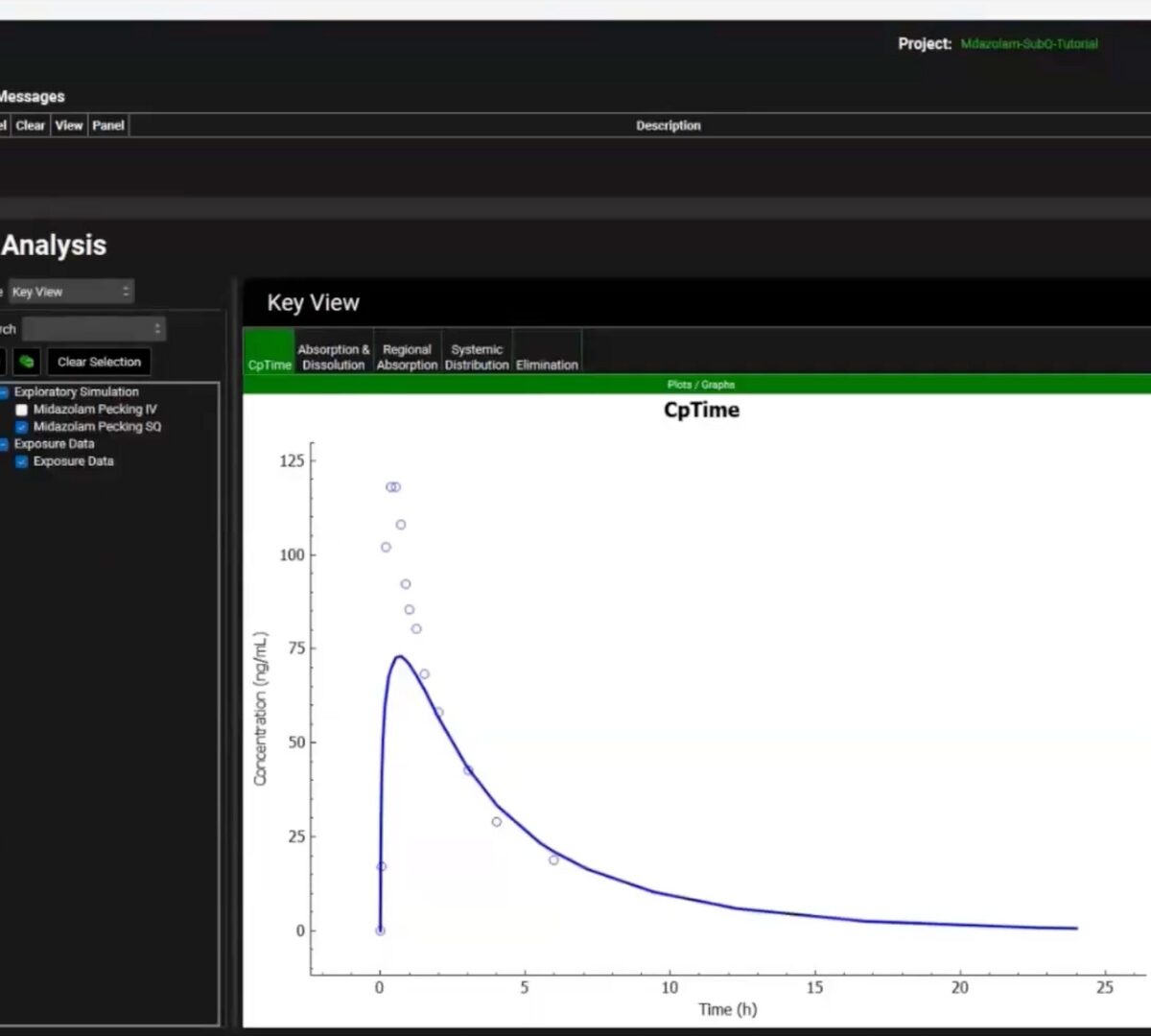
Get Under the Skin with GPX.2
If you’re working on the development of injectable therapies, then this webinar is for you
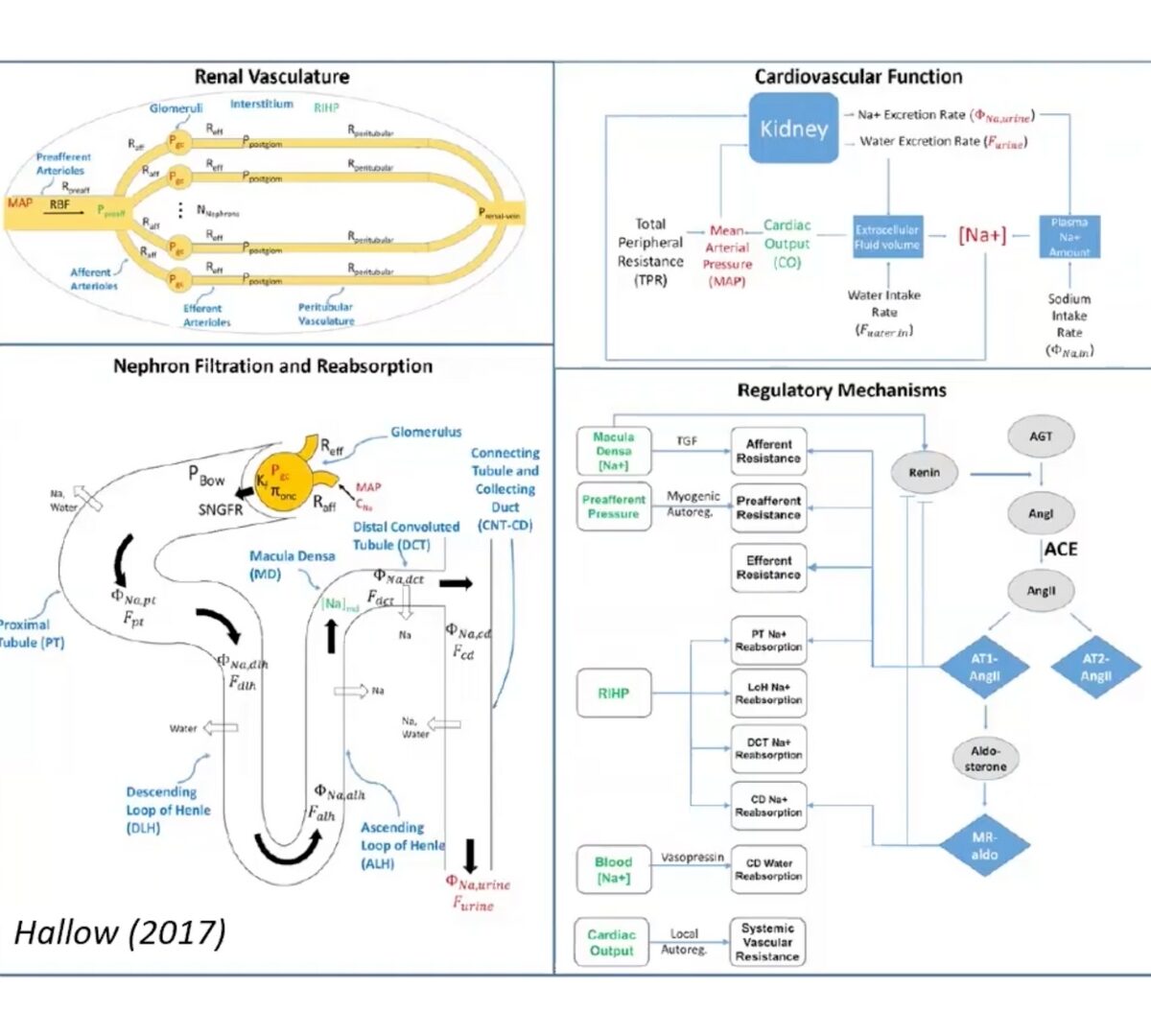
Advancing Kidney Safety: What’s New in RENAsym® 2A
Drug-induced kidney injury remains a significant concern in pharmaceutical development, with traditional methods often falling short in predicting renal toxicity across diverse compounds and patient populations.
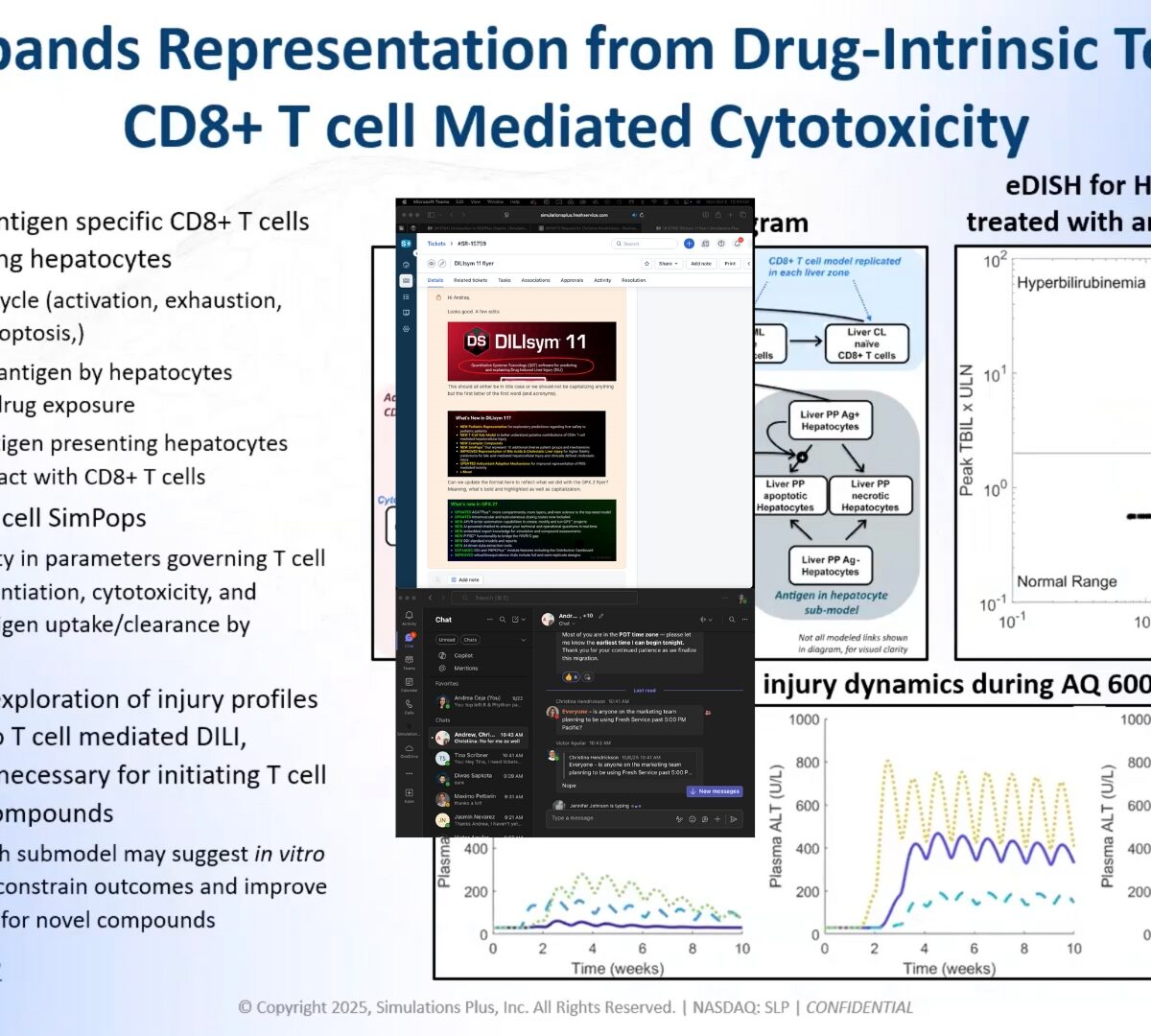
Unlocking the Power of Predictive Toxicology: What’s New in DILIsym® 11
Introducing DILIsym® 11, the latest evolution of the industry’s leading quantitative systems toxicology (QST) platform.
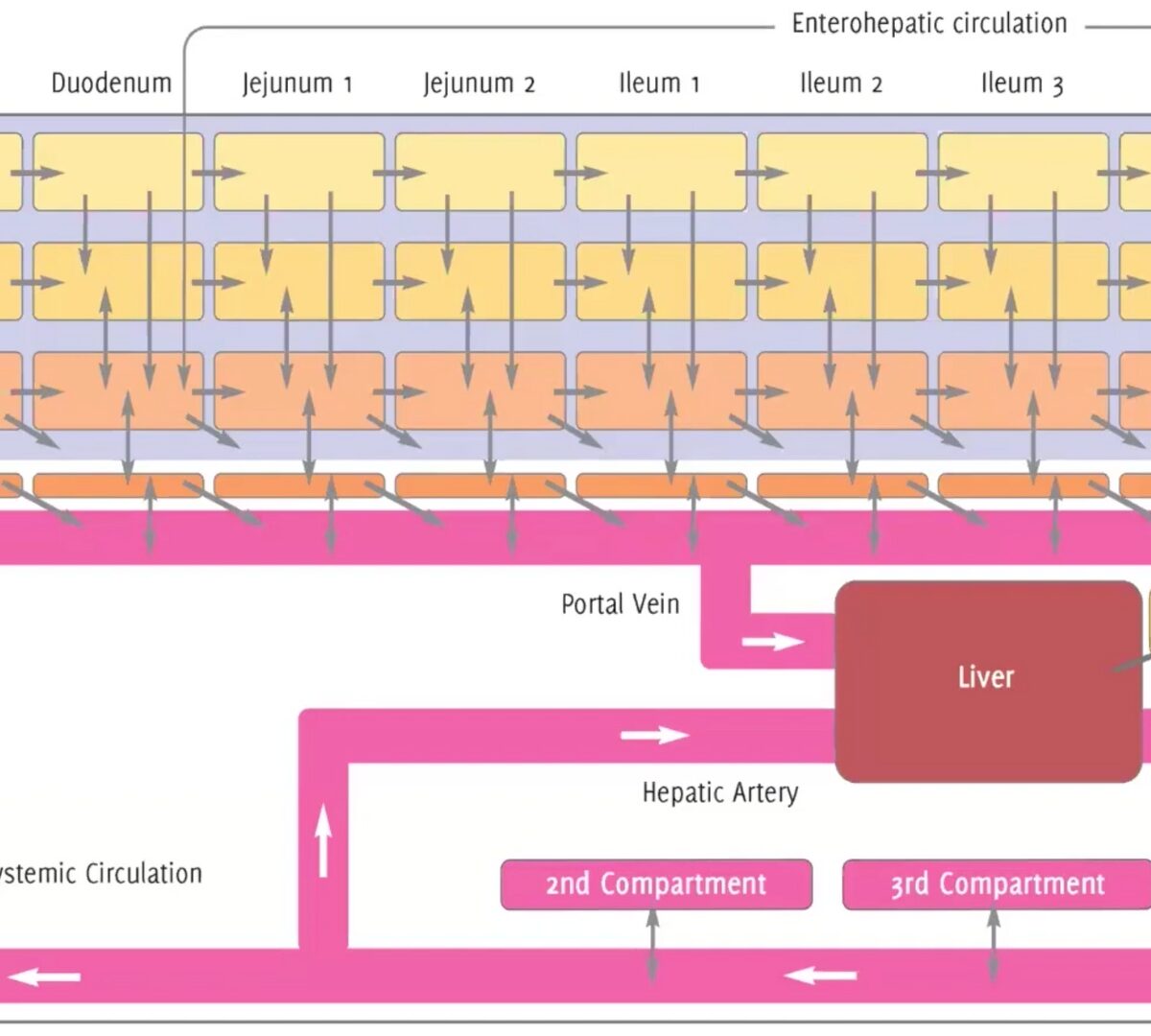
GastroPlus® X.2: The Deep Dive Webinar Series – How ACATPlus™ Can Be Used to Mechanistically Understand Local Drug Disposition in the Gastrointestinal Tract
Our ACAT™ model has been trusted by scientists for years, and the highly anticipated update—ACATPlus—is here.
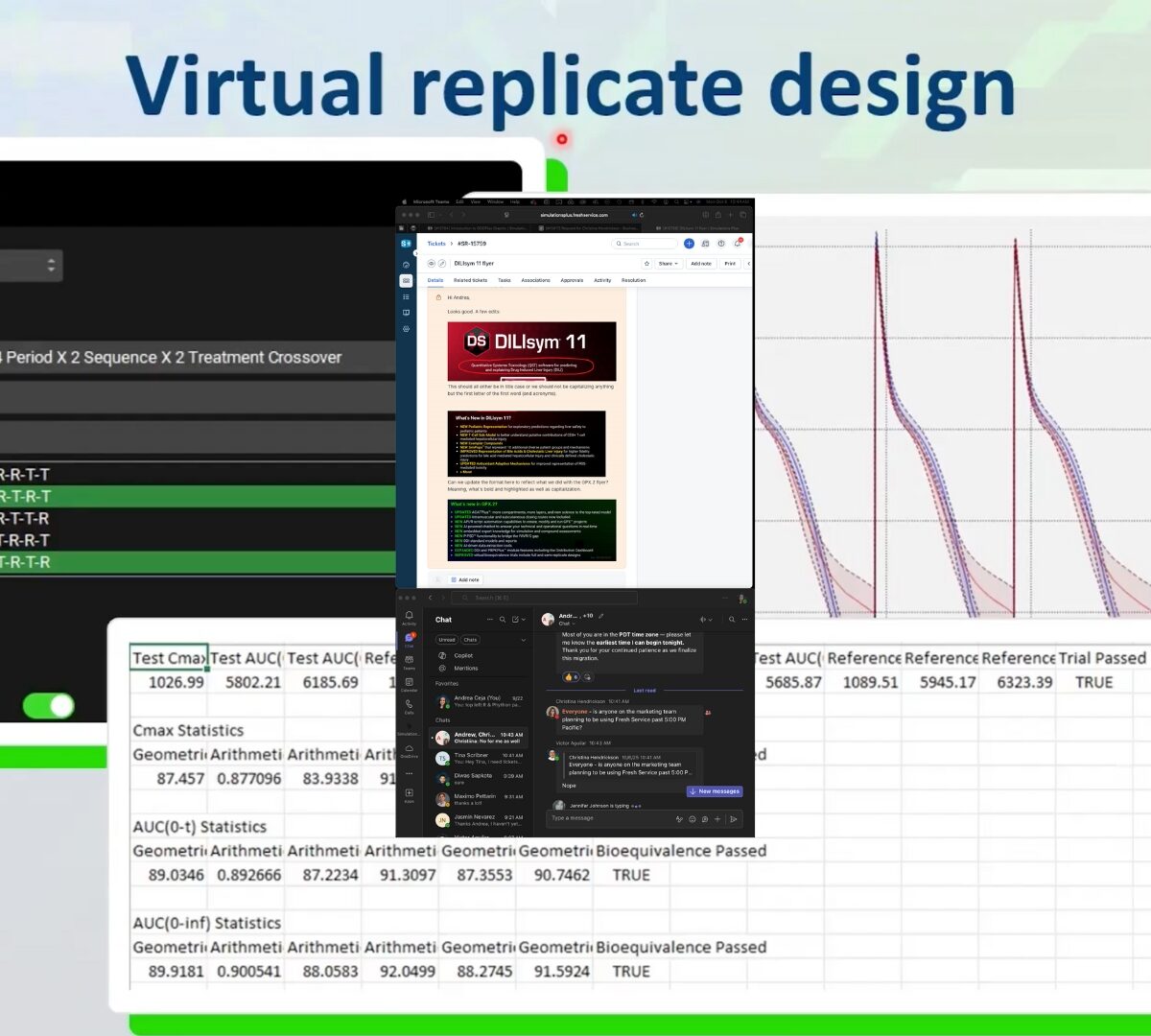
Introducing GastroPlus X.2: AI, Automation, Insights & More
Get a first look at game-changing new functionality that will revolutionize your PBPK/PBBM modeling.
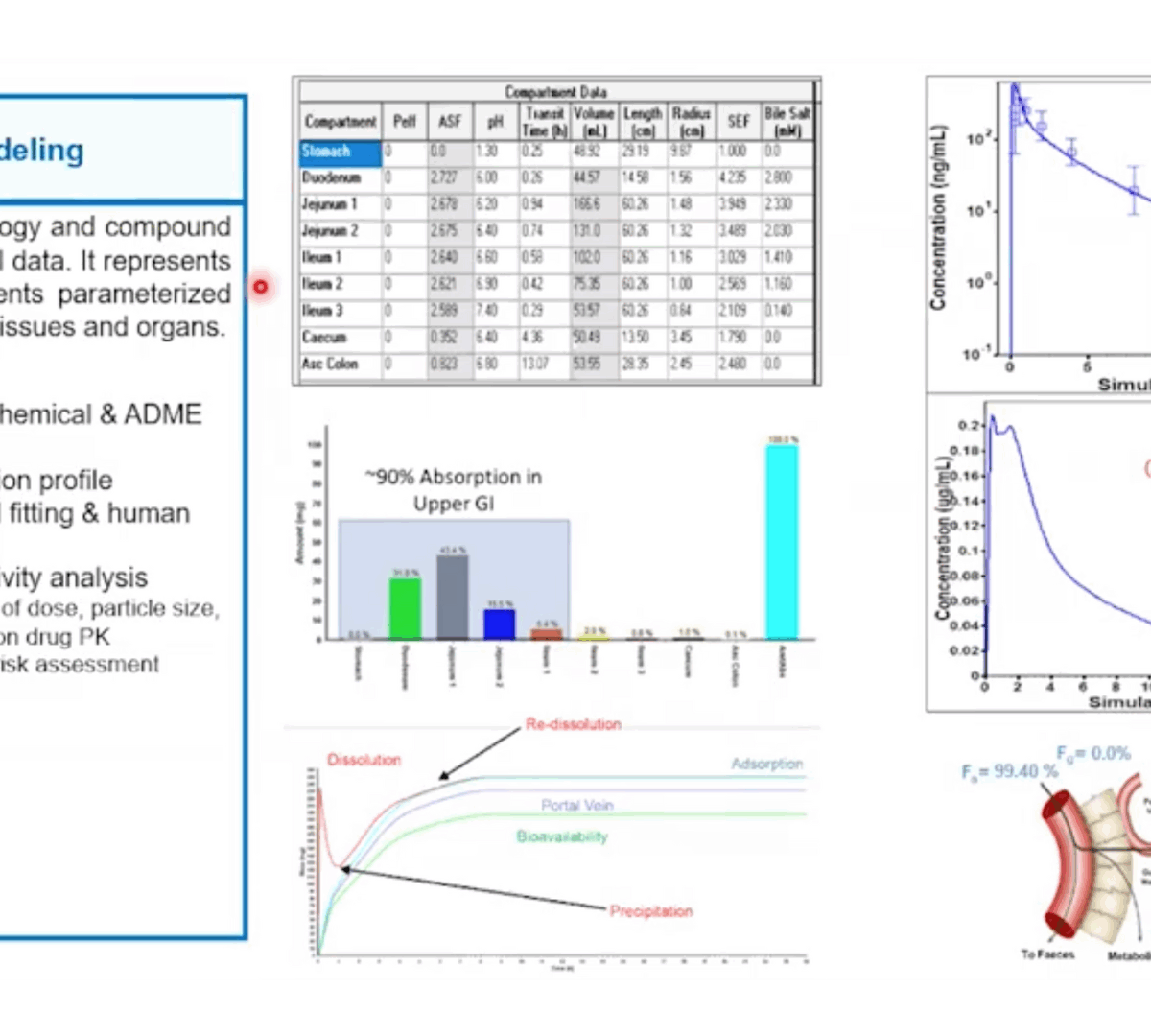
Can you spare 25mg? Optimizing Benchwork and Computational Approaches to Assess CMC Success
Drug discovery scientists often believe gram scale quantities of API are required to determine CMC properties like solubility, oral absorption, salt selection, and formulation strategy.

AI & Adaptive Learning: A New Era in Clinical Trial Optimization, Protocol Compliance and Risk Based Monitoring
In this webinar, Dr. Brad Stefanovic, VP, Head of Clinical Innovation at Simulations Plus, discusses how biotechs and big pharma alike can stretch their clinical trials dollars while optimizing key areas for compliance and success.
ADMET Predictor® 13: Predict & Build with Confidence
ADMET Predictor 13 is almost here—and in this webinar, you’ll see how it gives your organization the First-to-Invent Advantage! Drs. David Miller, Vice President, ADMET Cheminformatics, and Michael Lawless, Sr. Principal Scientist, Cheminformatics Solutions walk you through the latest version of the software...

Beyond the Lab: FDA’s Vision for Modeling a Future Without Animal Testing
The FDA has released a new roadmap outlining a path toward reducing—and ultimately replacing—animal studies in pharmaceutical development with new approach methodologies (NAMs), beginning with monoclonal antibodies.
Smarter Clinical Development: How to Use QSP to Maximize the Value of GLP-1 Agonists
As the market for GLP-1 agonists expands, biotech companies face both immense opportunity and fierce competition. To stand out in this evolving landscape and enhance the likelihood of acquisition or out-licensing, early-stage companies must develop a strategic, data-driven clinical development plan.
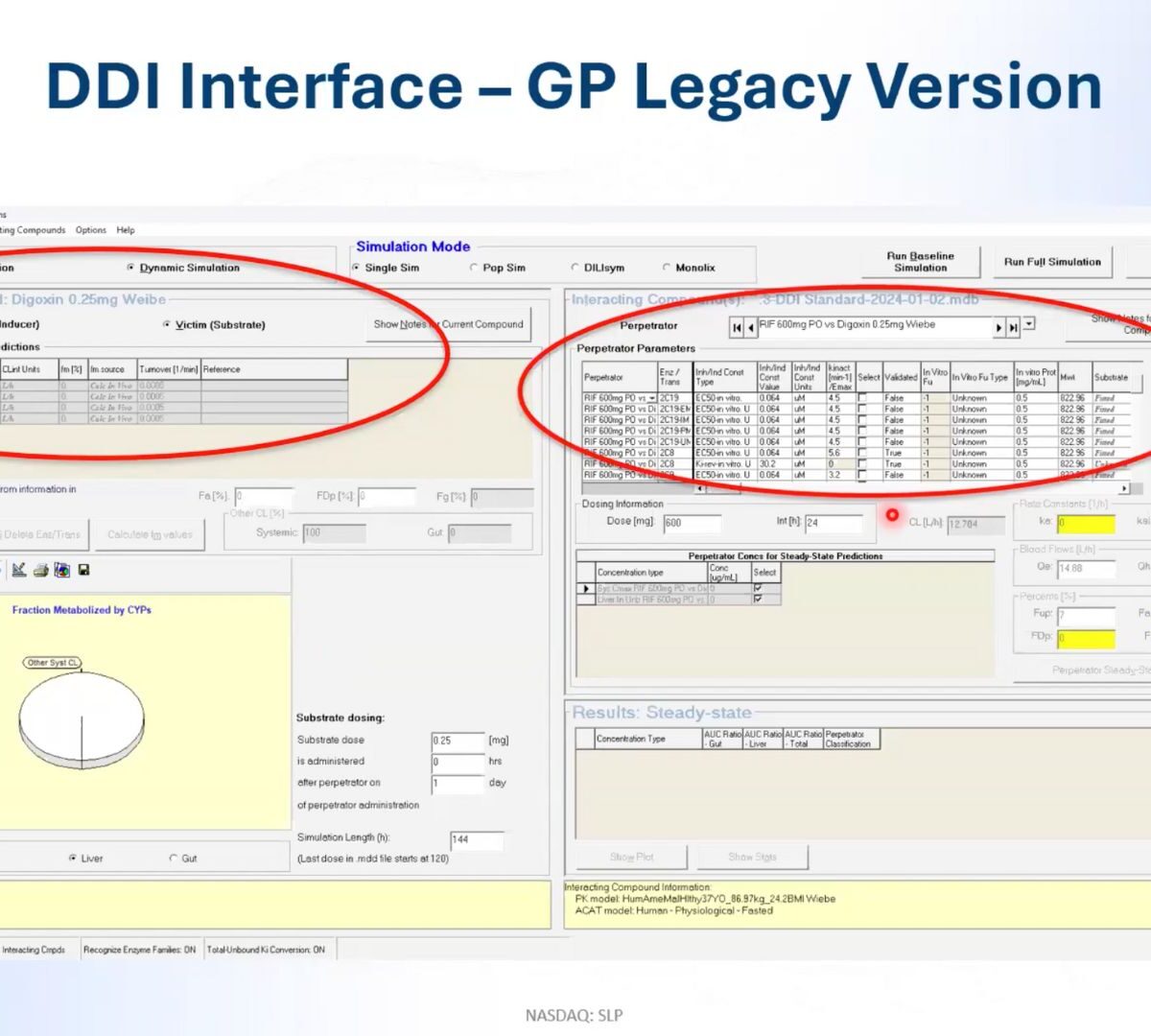
Mastering DDI Risk Assessment: Navigate Complexities of Transporter-Mediated DDIs
Transporters play a critical role in drug absorption, distribution, and elimination, and their involvement in drug-drug interactions (DDIs) can lead to altered drug concentrations and unexpected adverse effects that hamper the effectiveness of the treatment.
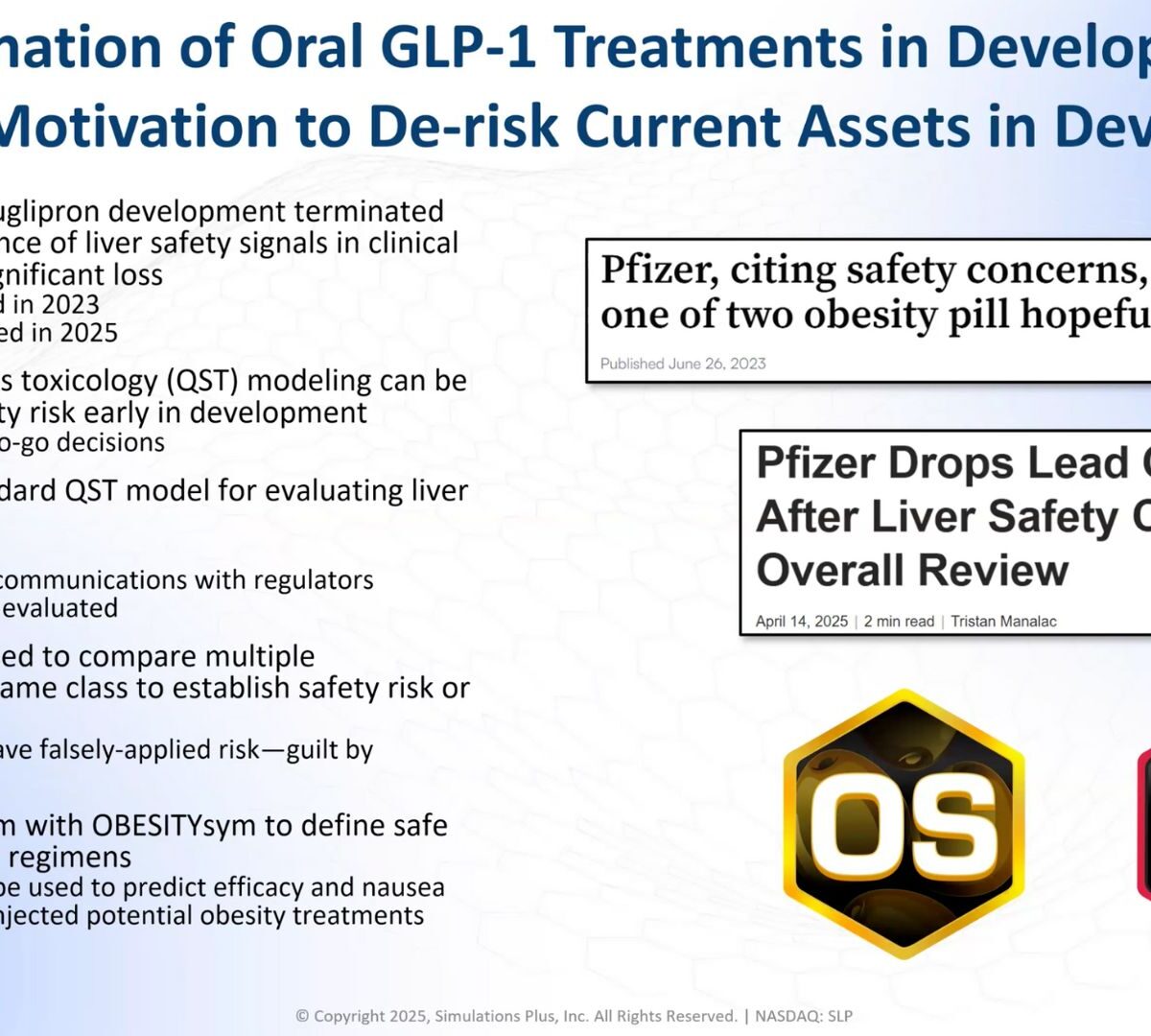
Focus Your Obesity Pipeline for Clinical Success: Delivering the Right Compound at the Right Dose for the Right Patient
Driving the development of your obesity drug assets efficiently is critical for success
Accelerating Small Molecule GLP-1 Agonist Optimization: AI-Driven Design Meets Mechanistic Modeling for a First-to-Invent Advantage
In a fast-paced industry with big budget competition, you need every tool and approach to succeed.
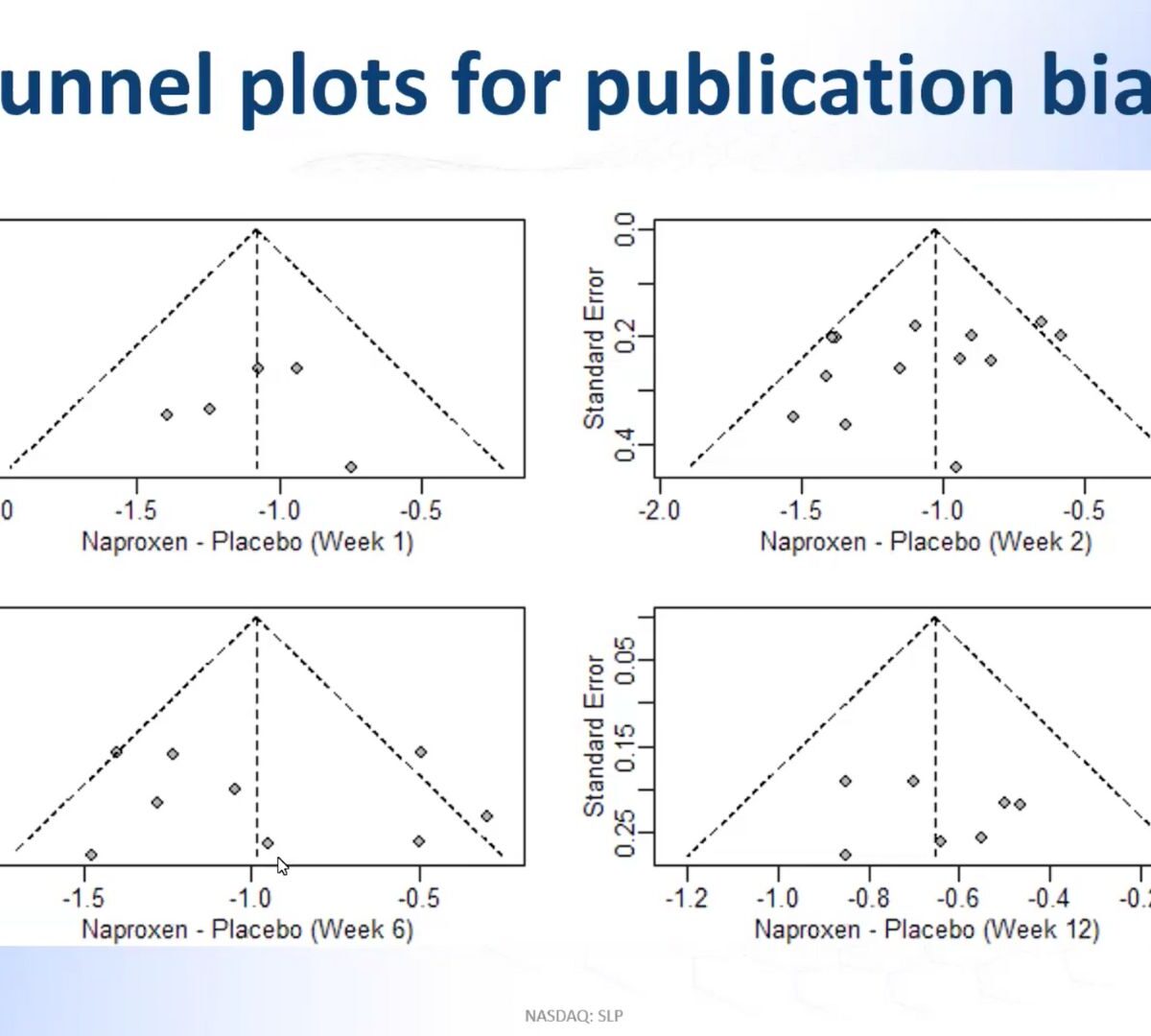
Longitudinal Model-Based Meta-Analysis (MBMA) Comprehensive MonolixSuite Tutorial with Case Studies
Model-based meta analysis (MBMA) informs key drug development decisions by integrating data, published or unpublished, from multiple studies.
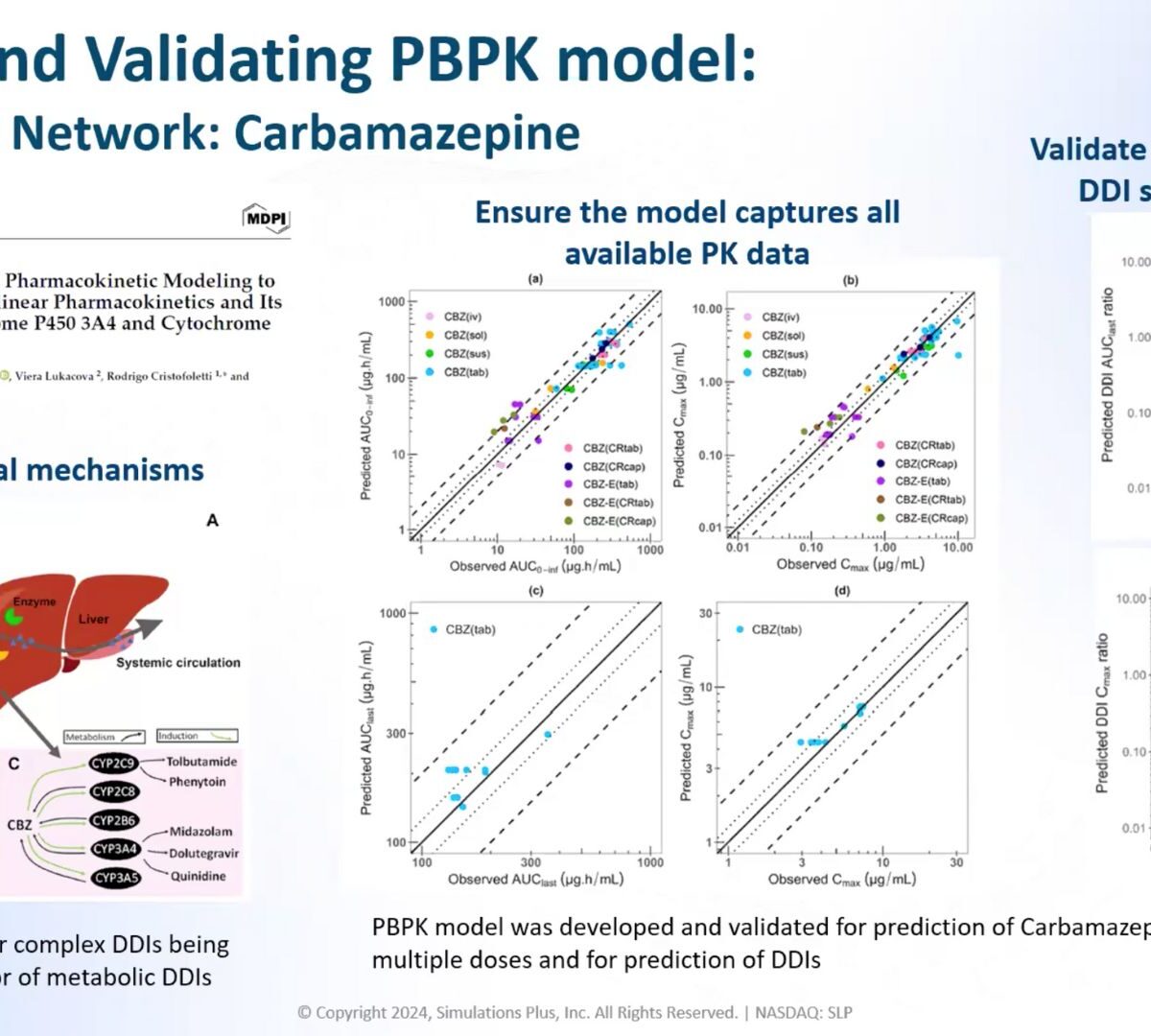
Mastering DDI Risk Assessment: Predict CYP enzyme-mediated DDIs with confidence
Understanding and predicting drug-drug interactions (DDIs) is crucial for ensuring patient safety and improving drug product development strategies.
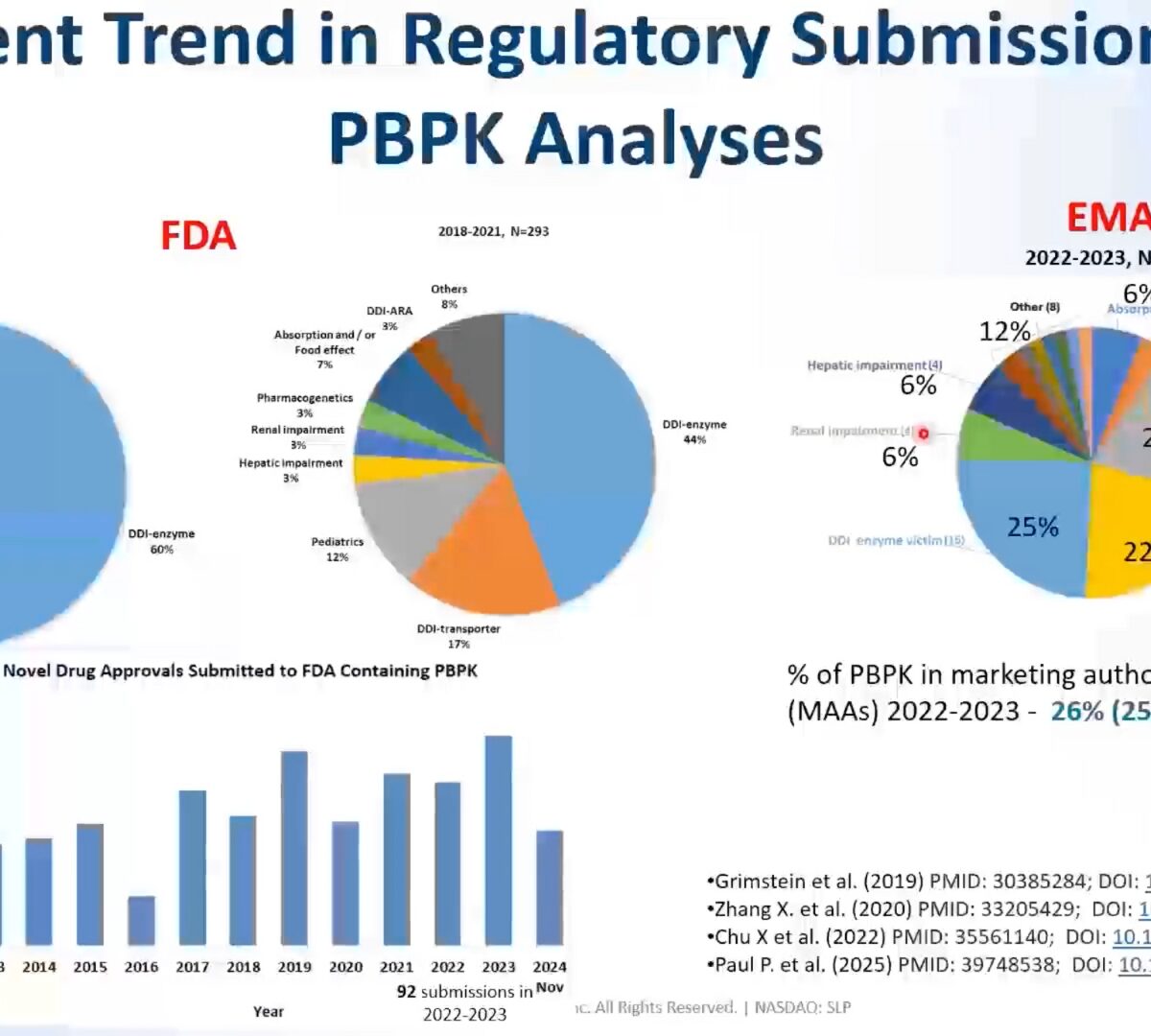
DDI Risk Assessment to Inform Your Label Optimize Your Program Timeline & Budget with PBPK Modeling
Every day, scientists in the pharmaceutical industry are tasked with meeting regulatory expectations while also minimizing budget spend by identifying efficiencies for faster development of safer, more effective drugs.
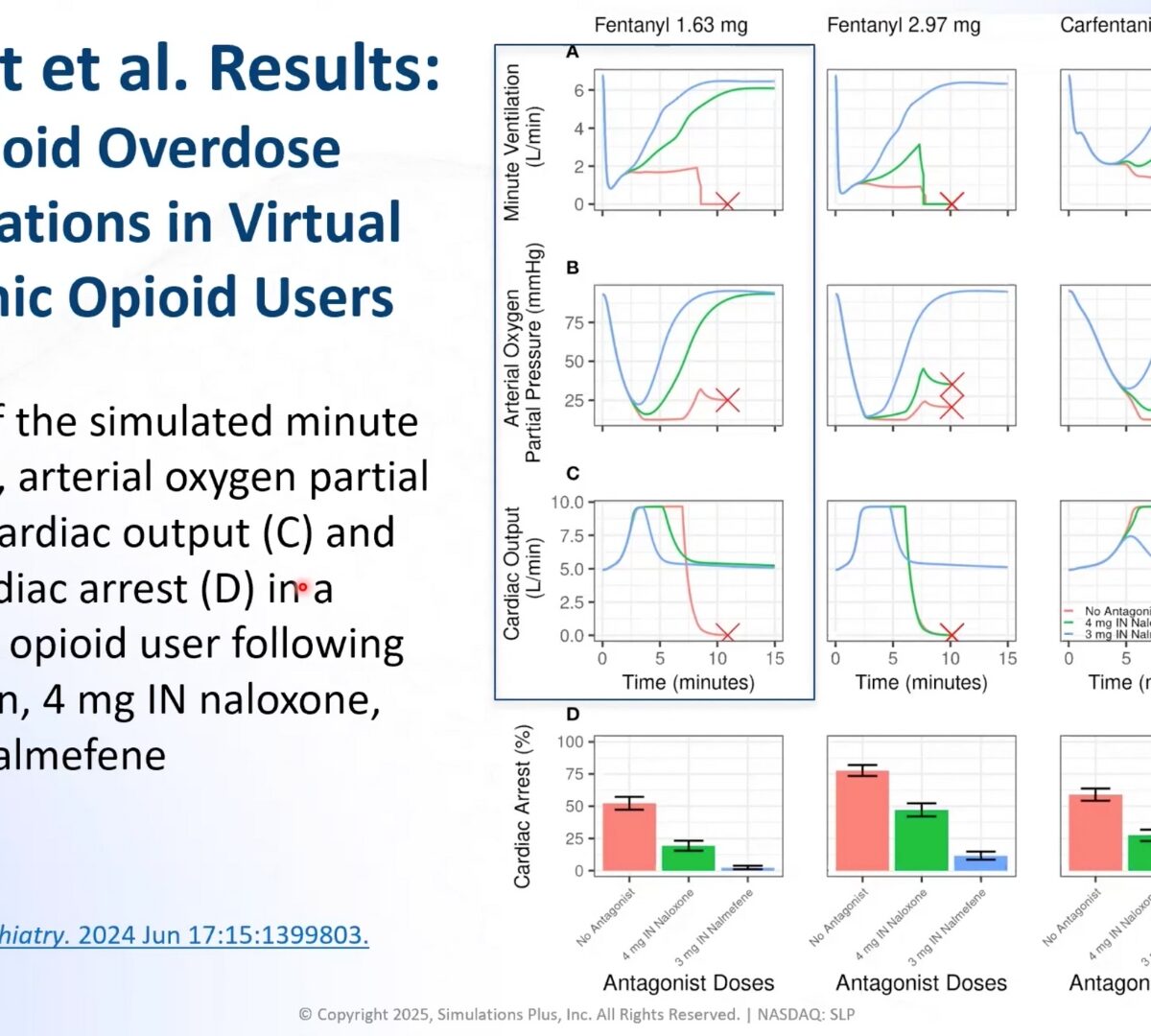
Evaluating the effectiveness of intranasal reversal agents using FDA’s translational model of opioid overdose
The number of opioid overdose deaths in the United States has continued to increase for more than two decades.

