The user interface in ADMET Predictor was completely rewritten for version 8. In this webinar, we demonstrate the new tools available in ADMET Predictor 8 using examples from various data sets, e.g., BACE1 inhibitors.
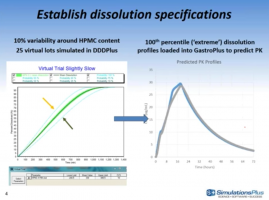
Reimagine the In Vitro Dissolution Experiment with DDDPlus™ 5.0…
In this webinar, case studies will be presented highlighting the use of DDDPlus and GastroPlus to optimize formulations and apply virtual 'lot-to-lot' variability effects to help establish dissolution specifications.

Unified dissolution / precipitation model and its use predicting absorption
This webinar will focus on the description of a novel mechanistic mathematical model developed to describe in vitro dissolution and precipitation data and how the model can be used in a wider PBPK framework...
Application of Cellular Permeability Simulation and PBPK Models to Capture
In this GastroPlus™ User Group webinar, we will discuss the validation of passive permeability estimates in MembranePlus™ based on molecular structure alone for a library of diverse compounds and how...

Applications of customized GastroPlus™ models
This GastroPlus™ User Group video explores how the ACAT model, with customized refinement of the model parameters or design of the model structure, can provide valuable insights to address various aspects of...
Analyzing the structural sensitivity of QSAR models using matched molecular
This video explores analyzing the structural sensitivity of QSAR models using matched molecular pairs using MedChem Studio™

Applications and considerations of drug exposure predictions in pediatric
This GastroPlus™ User Group webinar will focus on special considerations when performing pediatric PBPK modeling in GastroPlus™.
What’s New in ADMET Predictor™ 7.2?
Two new models, fraction unbound to liver microsomes and unbound human liver microsomal intrinsic clearance, were added to ADMET Predictor 7.2.
What’s New In GastroPlus™ 9.0?
This informative webinar covers the new features in the latest version of GastroPlus™ 9.0.
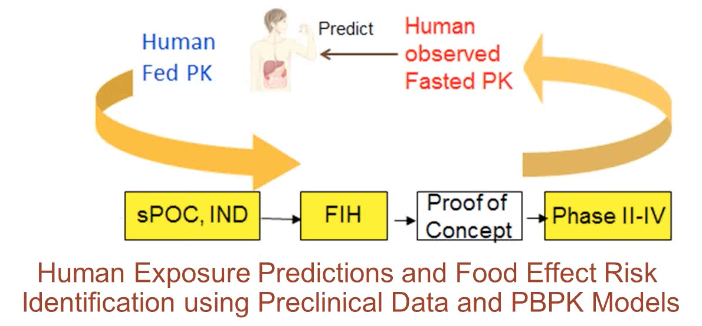
Human Exposure Predictions and Food Effect Risk Identification
This GastroPlus™ User Group webinar explores human exposure predictions and food effect risk identification using preclinical data and PBPK models.
Application of PBPK Modeling in Generic Drug Evaluation
This GastroPlus™ User Group/FDA webinar provides a few examples of modeling and simulation in the OGD for the purpose of addressing biopharmaceutical performance questions for orally administered generic drug products. View slides from this webinar.
Improving Bioavailability During Lead Optimization
In this presentation, Dr. Eric Martin of Novartis discusses improving bioavailability during lead optimization using global sensitivity analysis (GSA) of physiologically based pharmacokinetics.
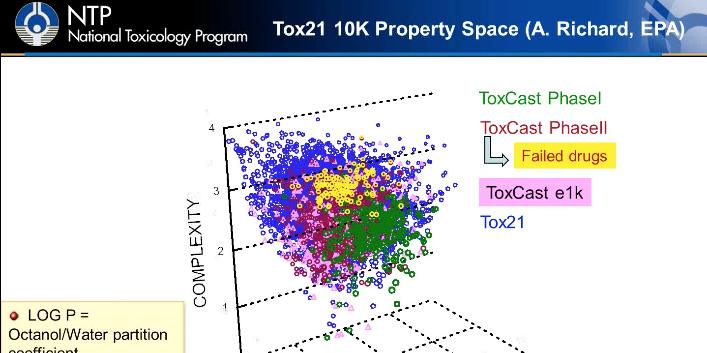
Analysis of the Tox21 10k Library with In Silico QSAR Models for Xenobiotic Metabolism and Toxicity
In this webinar Dr. Stephen Ferguson of the National Institutes of Environmental Health Sciences discusses in silico approaches to predict human xenobiotic metabolism and their potential for human toxicity.

What’s New in GastroPlus™ 8.6?
Learn about the new features in GastroPlus 8.6, how to build custom PBPK models for different disease states, and see a teaser of what's coming in version 9.0.

MembranePlus™ Webinar – Getting the Most out of Your In Vitro Permeability Studies
In this video, Dr. Viera Lukacova discusses how to get the most out of your in vitro permeability assays with MembranePlus™.

Identifying Structural Criteria for Potency using MedChem Studio™
This video shows how MedChem Studio™ can be used to analyze percent inhibition data from a high-throughput screening experiment in order to identify candidates for further optimization.

Cognigen Acquisition
This webinar is presented to provide details and answer questions concerning Simulations Plus' acquisition of Cognigen Corporation.

In silico design, synthesis, & testing of Cyclooxygenase (COX) inhibitors
Following the success of our earlier NCE project, which focused on the design of antimalarial molecules, we sought to utilize our ADMET Design Suite™ to design novel compounds that inhibit both...

GastroPlus™ Modeling Common Ion Effects and Enabled Formulations
Predictive mechanistic simulation of gastric dissolution and in vivo supersaturation and precipitation during oral absorption, using experimental parameters derived from in vitro measurements.

QbD and Biopharmaceutics – Use of Absorption Modeling
An informative online webinar that discusses the application of absorption modeling as a biopharmaceutics risk assessment tool to facilitate Quality by Design (QbD) arguments during formulation development.

