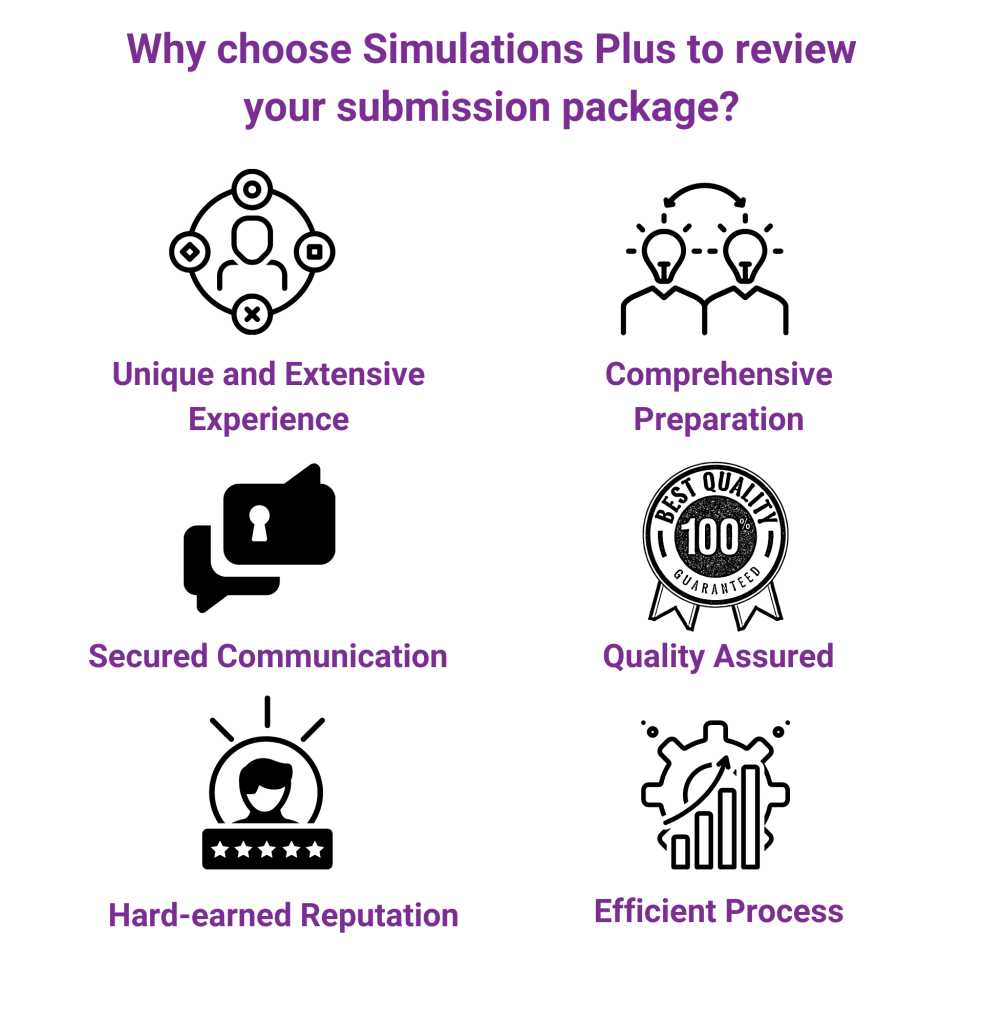
You have a fabulous pharmacometrics team. They’re smart, talented, experienced, and know PK/PD inside and out. But that doesn’t mean they don’t need a little help every once in a while. We can work with your team and lend a hand wherever you need that extra support: developing a rock-solid modeling and analysis plan, creating accurate analysis-ready datasets, providing an expert review and second set of eyes to quality check your modeling efforts, assisting with regulatory reporting and regulatory interaction support, or just as your overflow option when your team is fully booked. Basically, we’ll do the dirty work so you don’t have to.