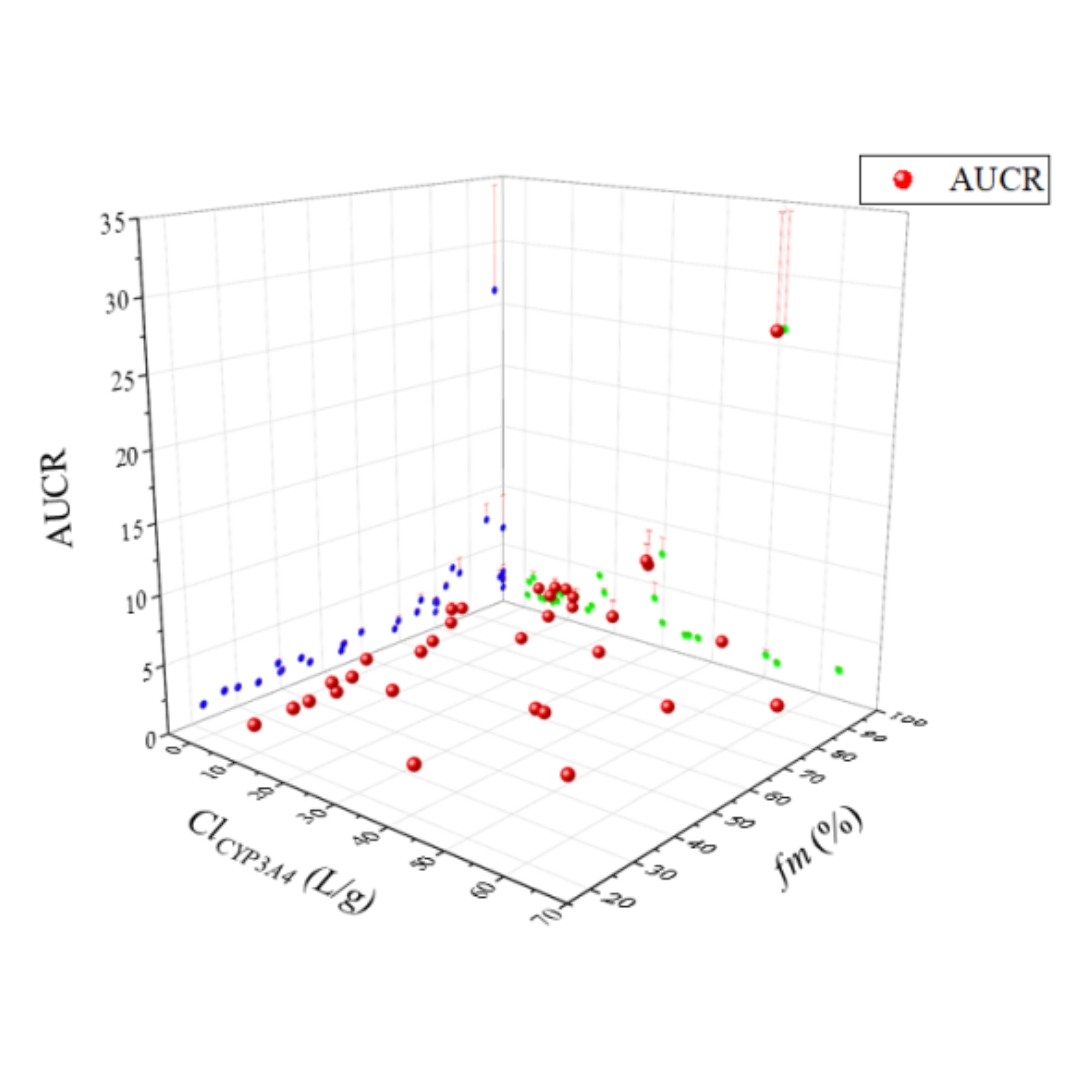
Enhancing the precision of drug-drug interaction (DDI) prediction is essential for mitigating potential drug interactions and enhancing drug safety and efficacy.

The Involvement of Hypoxia in the Response of Neuroblastoma Cells to the Exposure of Atorvastatin
Cancer is a set of complex diseases, being one of the leading causes of death worldwide.
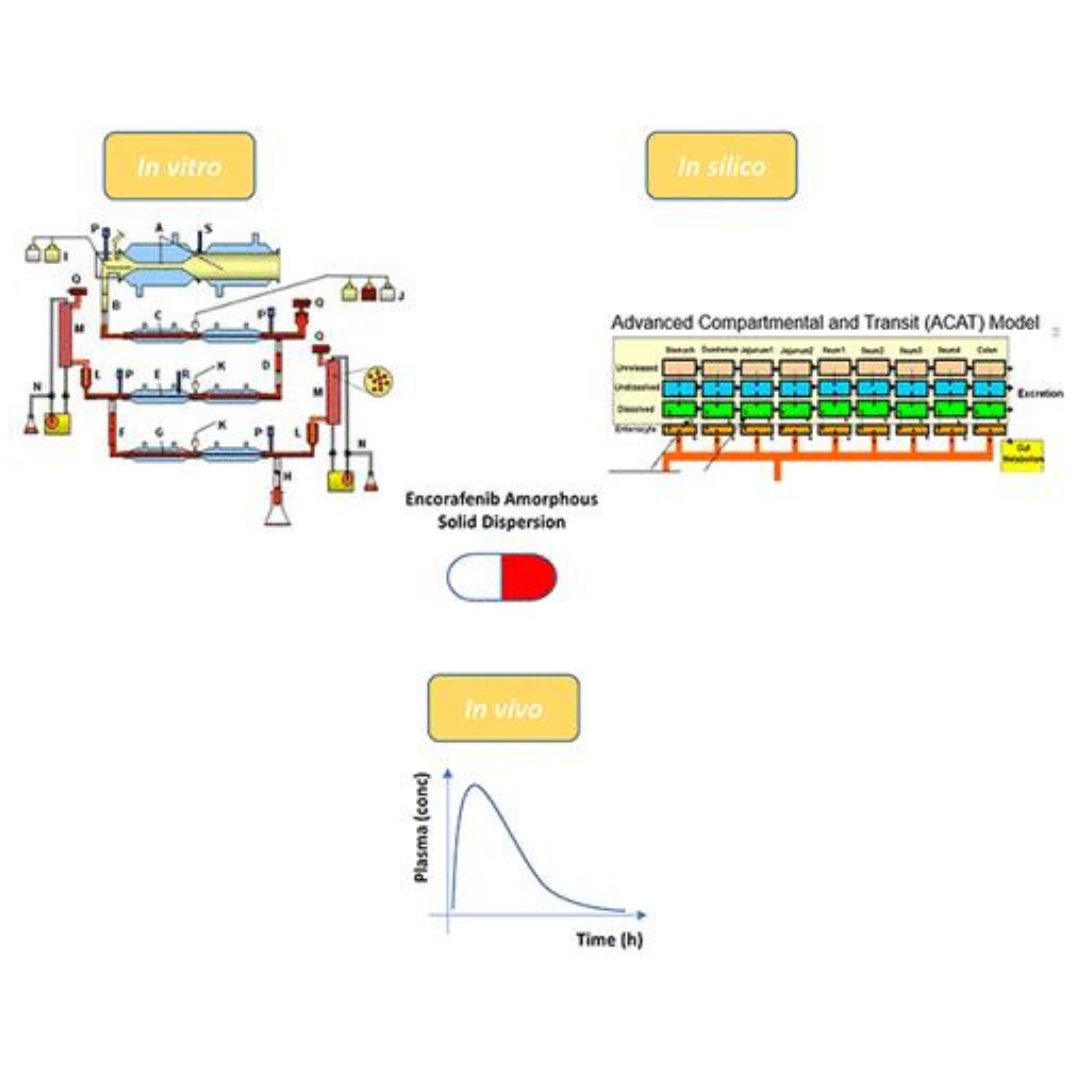
Effect of Food and a Proton-Pump Inhibitor on the Absorption of Encorafenib: An In Vivo–In Vitro–In Silico Approach
Encorafenib is a kinase inhibitor indicated for the treatment of patients with BRAF mutant melanoma and BRAF mutant metastatic colorectal cancer.

Therapy with Allogeneic SARS-CoV-2-specific T-cells for Persistent COVID-19 in Immunocompromised Patients
We administered SARS-CoV-2 VST under emergency IND to 6 immunocompromised patients with persistent COVID-19 and characterized clinical and virologic responses: three patients had partial responses after failing other therapies but then died.
An integrated computational approach to infer therapeutic targets from Campylobacter concisus and peptidomimetic based inhibition of its pyrimidine metabolism pathway
Campylobacter concisus is a commensal of the human oral flora that has been allied with persistent diarrhea and inflammatory bowel disease (IBD).

Ceftolozane/Tazobactam Probability of Target Attainment in Patients With Hospital-Acquired Pneumonia/ Ventilator-Associated Pneumonia
Probability of target attainment (PTA) analyses were conducted to support the recommended ceftolozane/tazobactam dosing regimens, adjusted for renal function, in patients with hospital-acquired/ventilator-associated...

Targeting Olokizumab-Interleukin 6 interaction interface to discover novel IL-6 inhibitors
The IL-6/IL-6R or IL-6/GP130 protein-protein interactions play a significant role in controlling the development of chronic inflammatory diseases, such as rheumatoid arthritis...
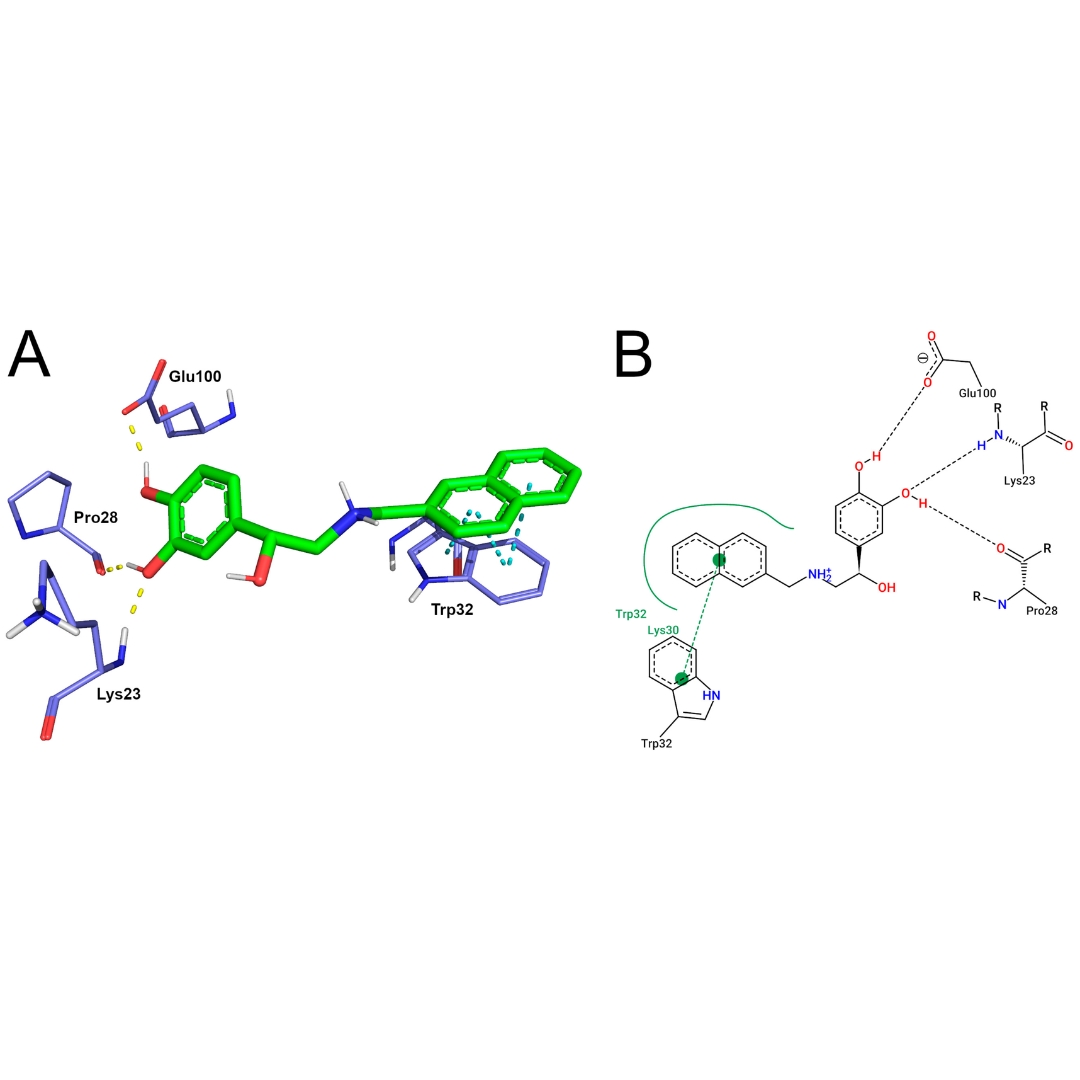
In Silico Analyses of a Promising Drug Candidate for the Treatment of Amyotrophic Lateral Sclerosis Targeting Superoxide Dismutase I Protein
Amyotrophic lateral sclerosis (ALS) is the most prevalent motor neuron disorder in adults, which is associated with a highly disabling condition
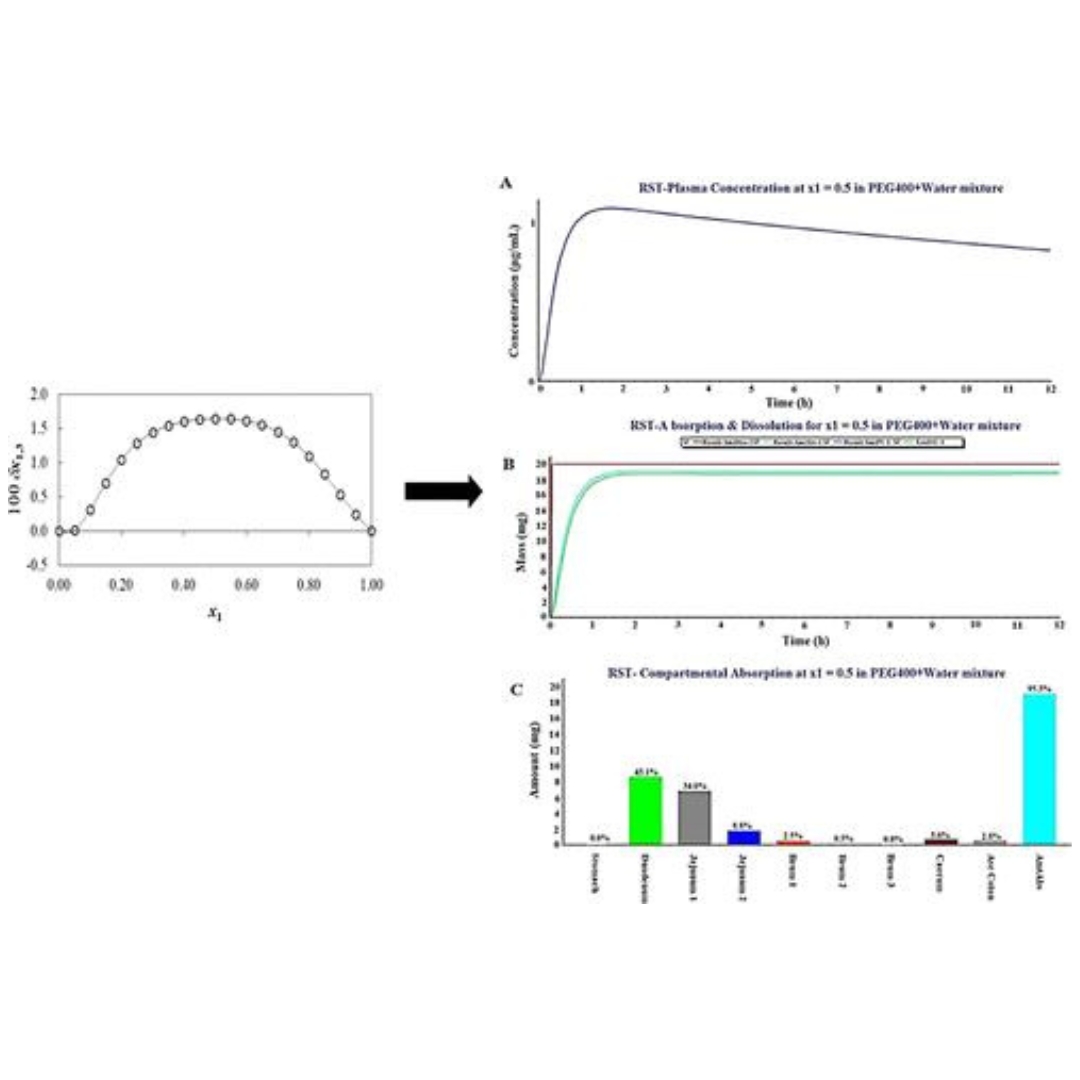
Preferential Solvation Study of Rosuvastatin in the {PEG400 (1) + Water (2)} Cosolvent Mixture and GastroPlus Software-Based In Vivo Predictions
Rosuvastatin (RST) is a poorly water-soluble drug responsible for limited in vivo dissolution and subsequently low oral systemic absorption (poor bioavailability).
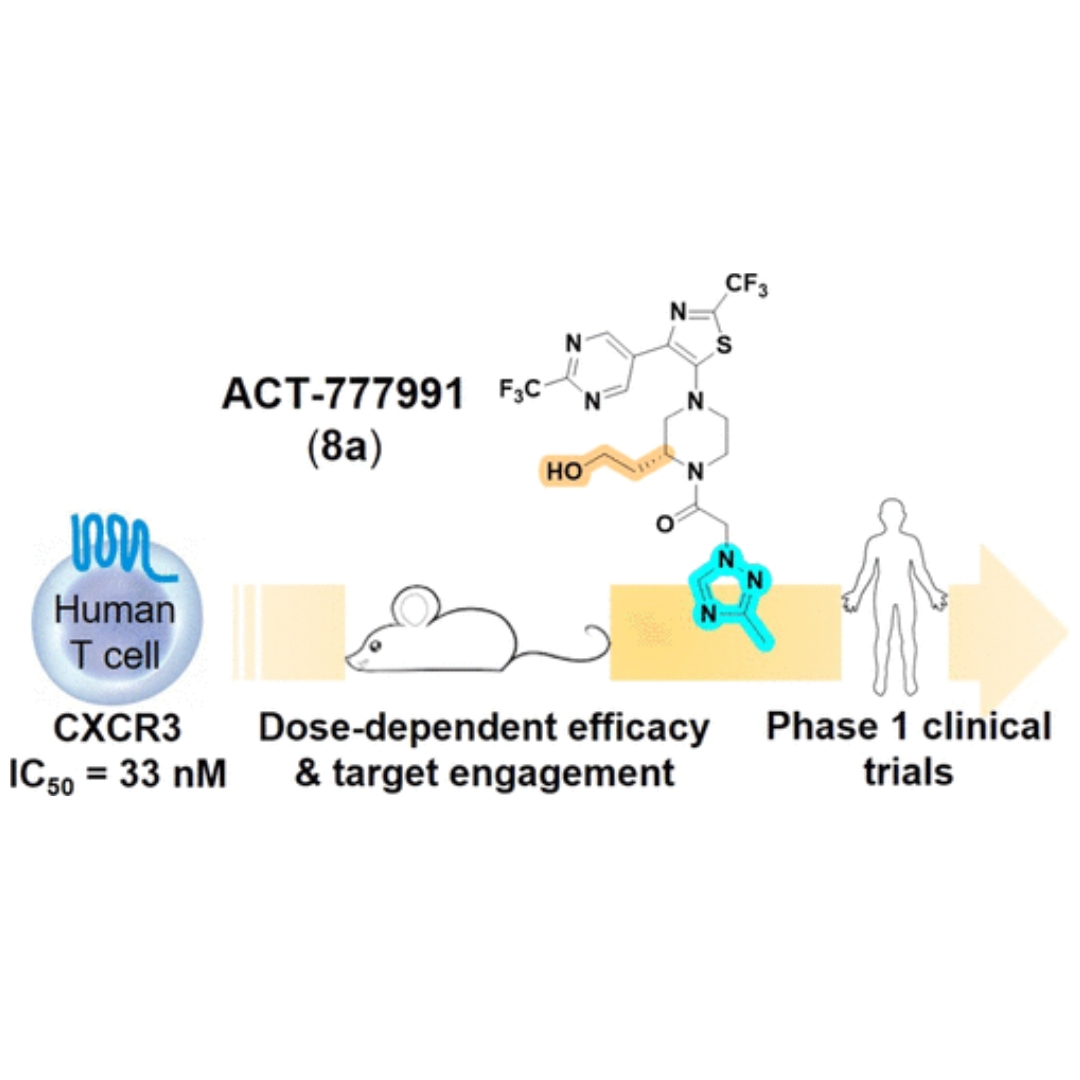
Discovery of Clinical Candidate ACT-777991, a Potent CXCR3 Antagonist for Antigen-Driven and Inflammatory Pathologies
The CXCR3 chemokine receptor is a G protein-coupled receptor mainly expressed on immune cells from the lymphoid lineage, including activated T cells.
![CMC development of [14C]-labeled sotorasib for the conduct of microtracer human ADME study](https://www.simulations-plus.com/wp-content/uploads/RC-1080x1080-22.jpg)
CMC development of [14C]-labeled sotorasib for the conduct of microtracer human ADME study
Human absorption, distribution, metabolism, and excretion (hADME) studies of new drugs are required for global regulatory filings.
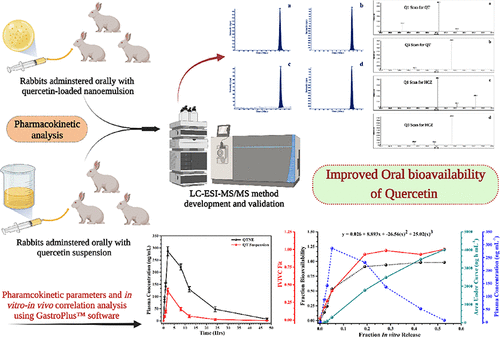
Liquid Chromatography–Electrospray Ionization Tandem Mass Spectrometry Estimation of Quercetin-Loaded Nanoemulsion in Rabbit Plasma: In Vivo–In Silico Pharmacokinetic Analysis Using GastroPlus
In the present study, we developed and validated a rapid, specific, sensitive, and reproducible liquid chromatography–electrospray ionization tandem mass spectrometry method...

Physiologically Based Pharmacokinetic Modelling to Predict Pharmacokinetics of Enavogliflozin, a Sodium-Dependent Glucose Transporter 2 Inhibitor, in Humans
Enavogliflozin is a sodium-dependent glucose cotransporter 2 (SGLT2) inhibitor approved for clinical use in South Korea.

Computational Analysis and Experimental Testing of the Molecular Mode of Action of Gatastatin and Its Derivatives
Given its critical role in cell mitosis, the tubulin chain represents a viable chemotherapeutic target to solve the specificity issues associated with targeting...
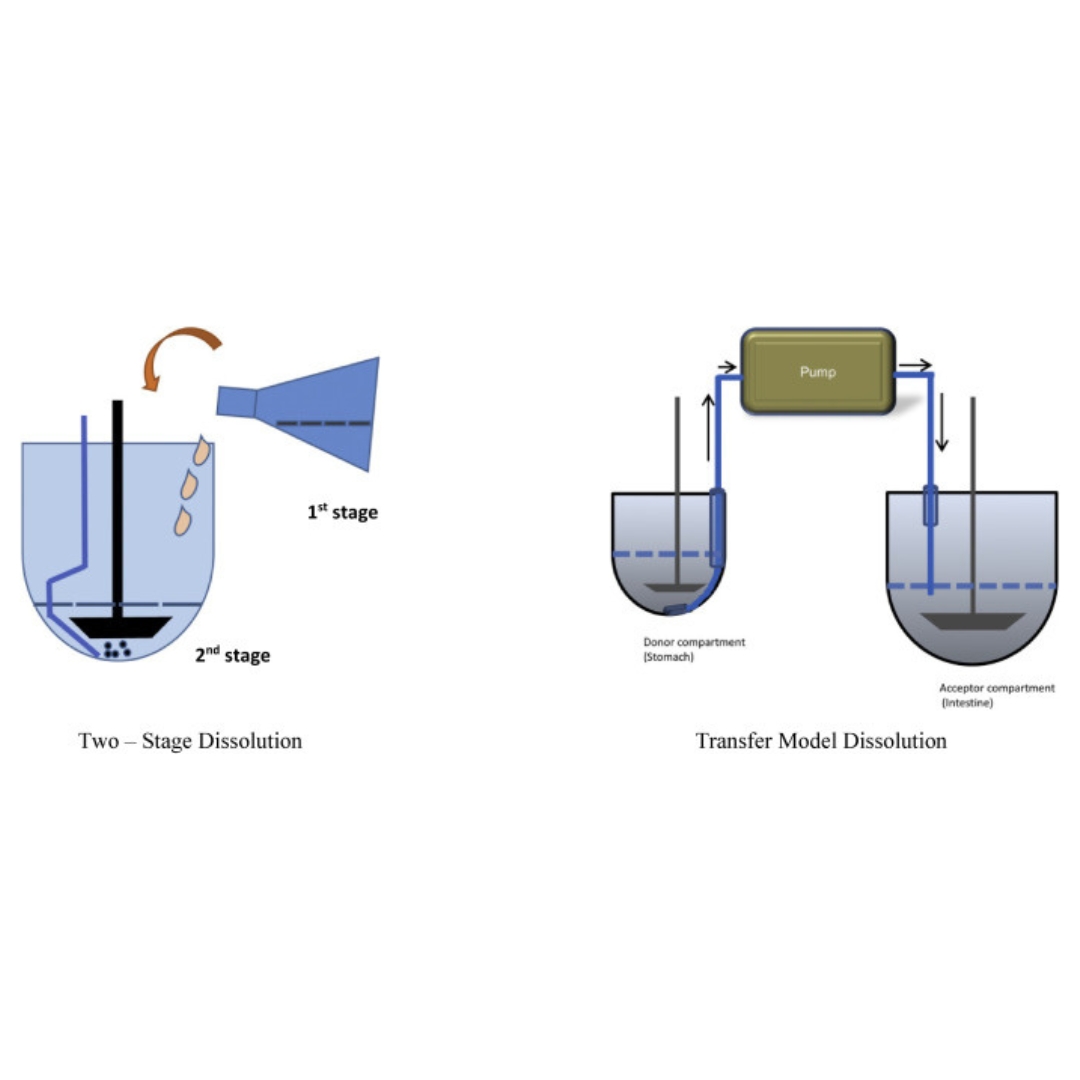
Evaluation and prediction of oral drug absorption and bioequivalence with food-druginteraction
This article reviews the impacts on the in vivo prediction of oral bioavailability (BA) and bioequivalence (BE) based on Biopharmaceutical classification systems (BCS)...
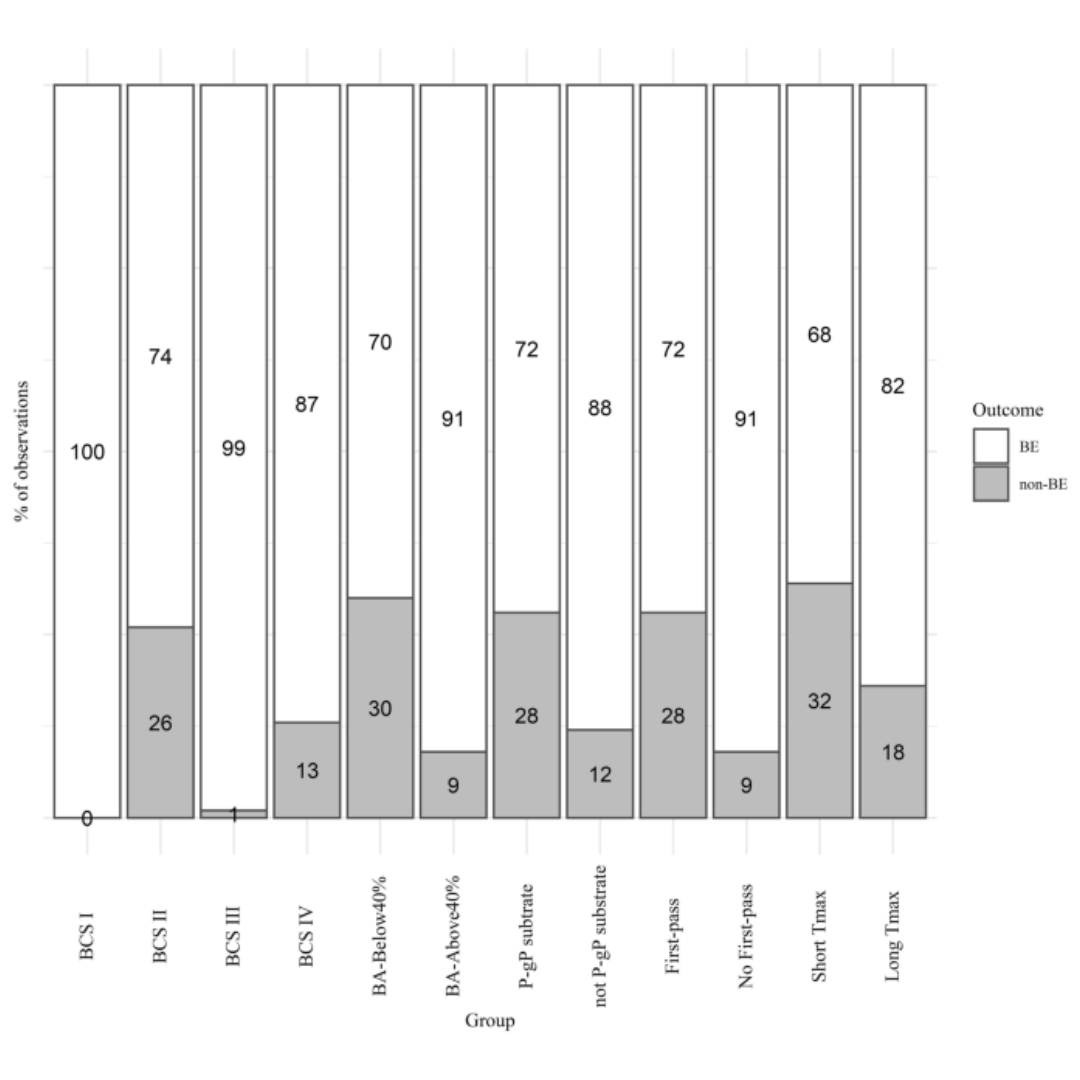
Predictive Potential of BCS and Pharmacokinetic Parameters on Study Outcome: Analysis of 198 In Vivo Bioequivalence Studies
Understanding predictive potential of parameters to perform early bioequivalence (BE) risk assessment is crucial for good planning and risk mitigation during product...
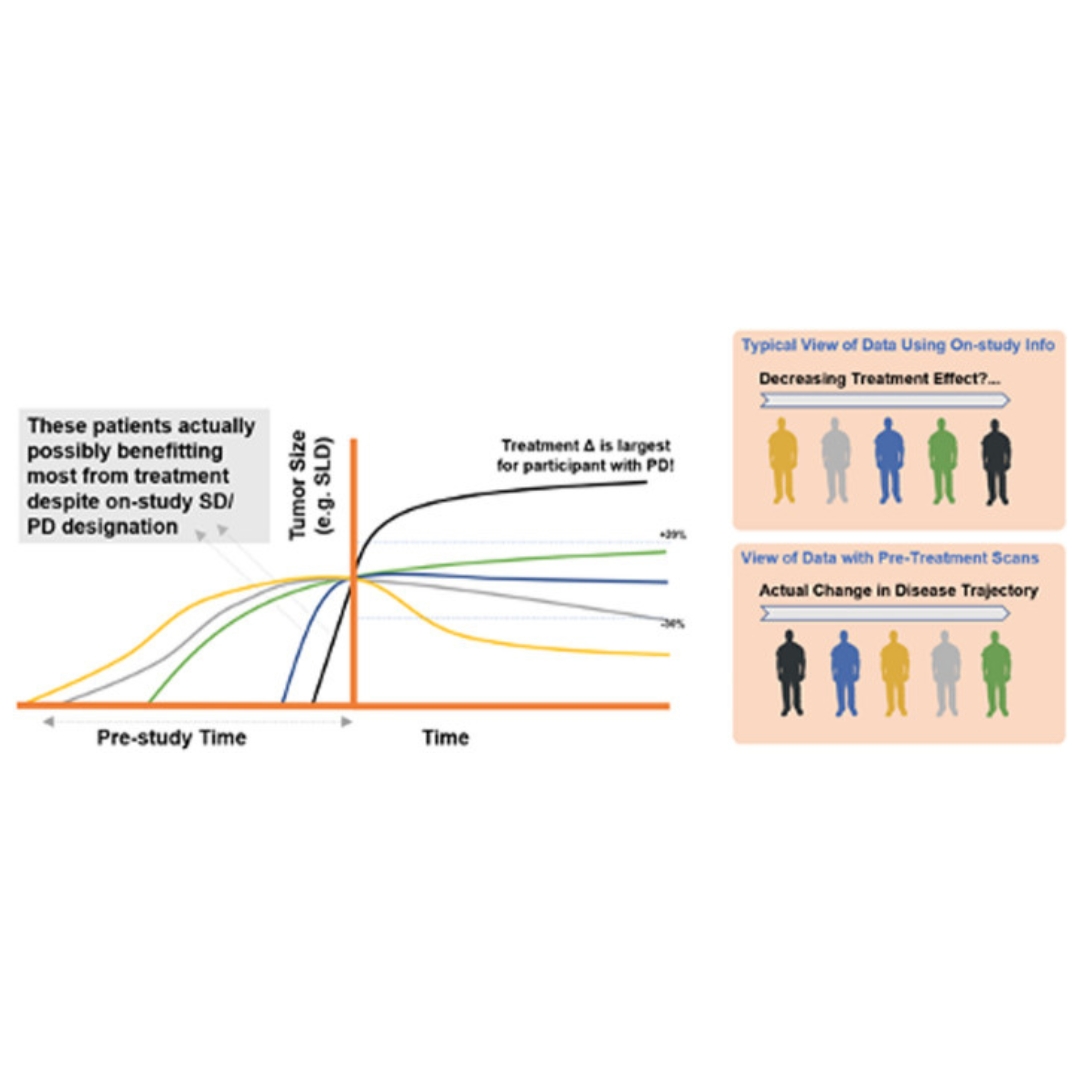
A comprehensive regulatory and industry review of modeling and simulation practices in oncology clinical drug development
Exposure-response (E-R) analyses are an integral component in the development of oncology products.
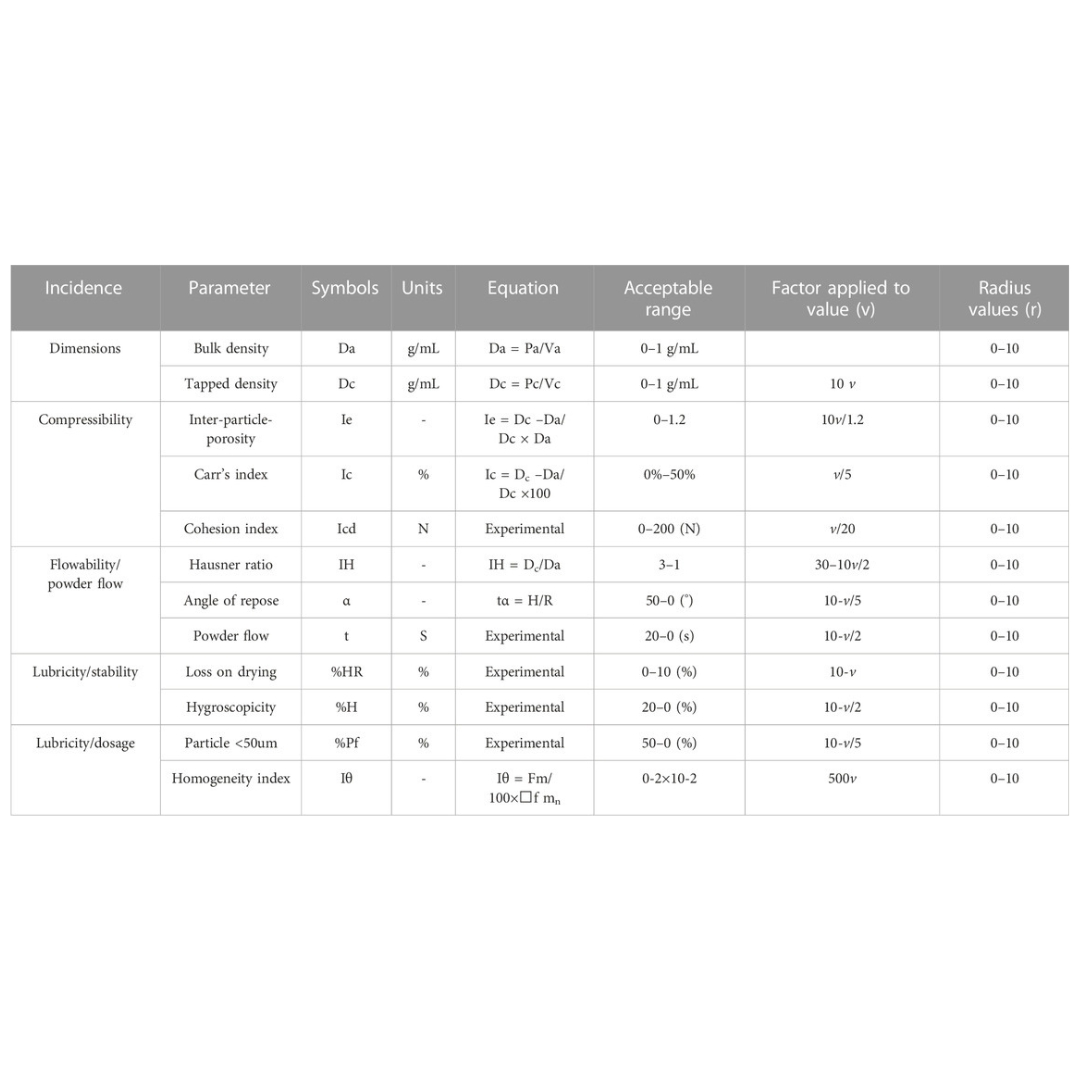
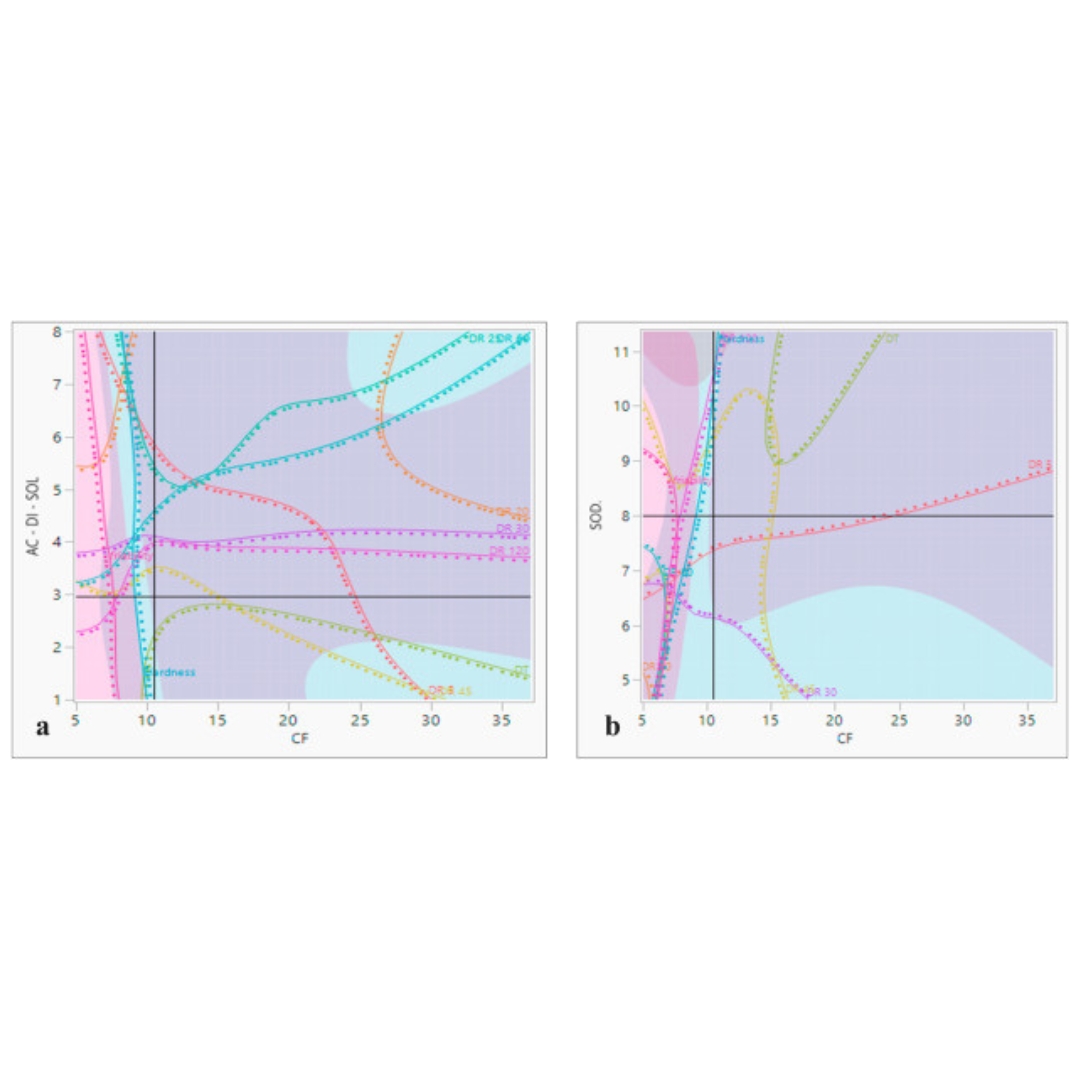
SeDeM expert system with I-optimal mixture design for oral multiparticulate drug delivery: An encapsulated floating minitablets of loxoprofen Na and its in silico physiologically based pharmacokinetic modeling
Introduction: A SeDeM expert tool-driven I-optimal mixture design has been used to develop a directly compressible multiparticulate based extended release minitablets...
Physiologically Based Pharmacokinetic Modeling Characterizes the Drug-Drug Interaction Between Saxagliptin and Rifampicin in Patients With Renal Impairment
The aim of the present study is to develop physiologically based pharmacokinetic (PBPK) models for saxagliptin and its active metabolite, 5-hydroxy saxagliptin...
A hybrid framework of artificial intelligence-based neural network model (ANN) and central composite design (CCD) in quality by design formulation development of orodispersible moxifloxacin tablets: Physicochemical evaluation, compaction analysis, and its in-silico PBPK modeling
The objective was to apply the CCD-ANN system to design a QbD-based orodispersible tablet (ODT) of Moxifloxacin. The data sets of the trial formulations...