Acetaminophen has a long history of safe use at therapeutic doses, but can cause liver injury at very high doses.

Synergy Between Two Mechanisms of Action Contributes to Species Differences in the Liver Safety Profile for PF-04895162
PF-04895162 (ICA-105665), a drug in development for the treatment of epilepsy, was terminated after transaminase elevations (up to grade...

Quantitative Systems Toxicology Modeling of Cisplatin Nephrotoxicity Using in vitro Assays of Proximal Tubule Epithelial Cells for Mechanistic Toxicity Pathways
Cisplatin-induced nephrotoxicity results in acute kidney injury (AKI) and is caused by various cellular mechanisms, including...

Using in silico-in vitro to in vivo Extrapolation (IS-IVIVE) to Predict the Oral Dose Required to Activate the Aryl Hydrocarbon Receptor (AhR)
Activation of AhR can have toxic effects on mammalian hosts

Mechanistic Modelling of the Linkage Between Proximal Tubule Cell Sublethal Injury and Tubular Sodium Reabsorption Impairment
Sublethal renal epithelial cell injury, a key manifestation of drug-induced acute kidney injury (AKI), is characterized by loss of brush border and cellular polarity of proximal tubular cells (PTCs).

Evaluating the Nephrotoxicity of Exemplar Compounds Using a Mechanistic Model of Drug-Induced Acute Kidney Injury
Drug-induced nephrotoxicity is a common source of acute kidney injury (AKI) and a condition that complicates clinical outcomes of vulnerable patients.

Mechanistic Modeling Aids in the Interpretation of Alanine Aminotransferase Elevations Associated with Clinical Ischemic Liver Injury
DILIsym® software can use serial serum alanine aminotransferase (ALT) assessments to predict hepatocyte loss (HL) and corresponding changes in total bilirubin (TBIL) due to...

Physiologically Based Pharmacokinetic Model for Voriconazole and Prediction of its Interactions with Midazolam and Alfentanil
Voriconazole (formerly known as UK-109,496), is a second-generation triazole antifungal agent widely used in the treatment of invasive fungal infections.

Exploring Clinical Relevance of Dissolution Testing by Physiologically Based Biopharmaceutics Modeling (PBBM)
In vitro dissolution testing, if reflective of in vivo drug release/absorption, is considered a surrogate for in vivo drug performance.

Predicting Shrinkage of Individual Parameters in More Complex NLME Models Using Bayesian Fisher Information Matrix
When data are sparse, parameters derived from a non-linear mixed effects model analysis...

Synergy Between Two Mechanisms of Action Contributes to Species Differences in the Liver Safety Profile for PF-04895162
The Purpose is to better understand the mechanisms underlying the apparent species differences, between rat and human, when evaluating the liver safety of compound PF-04895162.

Development of a Physiologically Based Pharmacokinetic (PBPK) Model for Intra-articular Delivery
Understanding local concentrations in intra-articular tissues and fluids such as cartilage, synovial membrane, and synovial fluid are a valuable tool to predict potential...

Efavirenz Physiologically Based Pharmacokinetic Model Development and Validation as a Moderate CYP3A4 Inducer for Drug-Drug Interaction Predictions
Efavirenz is an antiretroviral medication used to treat and prevent HIV/AIDS.

The Effect of the Local Tissue Response on the Pharmacokinetics of Long-Acting Injectable Formulations
Modeling consequences of localized chronic inflammation in tissue on drug diffusion and exposure caused by prolonged therapy with long-acting formulations.

Modeling and Simulation of the Local Tissue Response to the Long-acting Injectable Formulations
Recently, long-acting injectable (LAI) drug formulations have attracted much attention for prolonged drug exposure from weeks to several months.

Adapting a quantitative systems toxicology model of mitochondrial dysfunction in liver to kidney
Kidney, as a major excretory organ, is exposed to high levels of drugs and their metabolites.
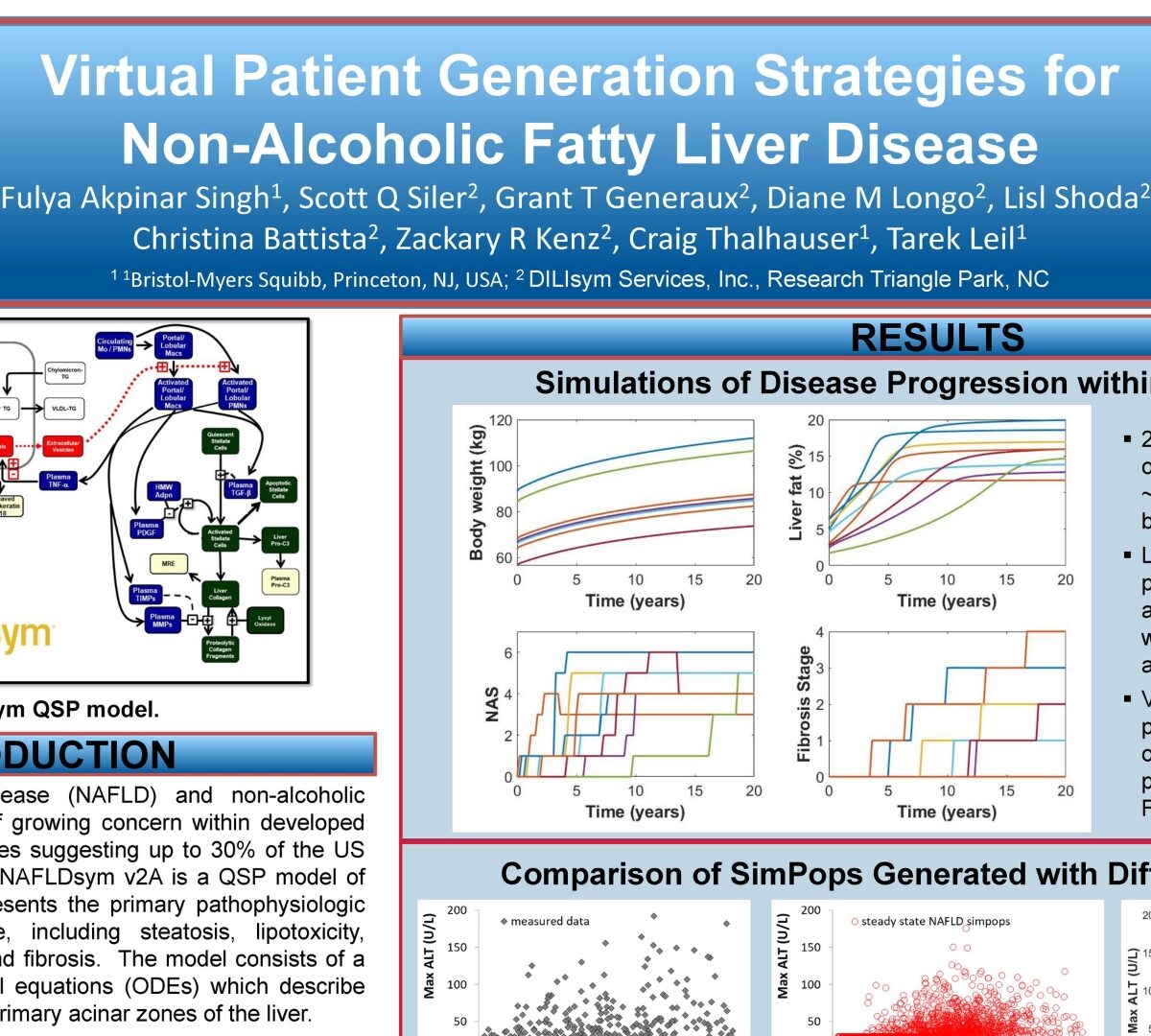
Virtual Patient Generation Strategies for Non-Alcoholic Fatty Liver Disease
Non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) are of growing concern within developed countries, with recent estimates suggesting up to 30% of the US population may be affected.

Mechanistic Analysis of Cisplatin-Induced Acute Kidney Injury Using Quantitative Systems Toxicology Modeling
Acute kidney injury is a common side effect of cisplatin chemotherapy.

The solubility-absorption trade-off in using solubilizers: mechanistic PK simulations of progesterone with explicit cyclodextrin
Cyclodextrins improve solubility of poorly soluble lipophilic drugs due to 1:1 complexation in their nonpolar interior cavity.

Physiologically Based Pharmacokinetic Modeling of Rosuvastatin and Prediction of Transporter-Mediated Drug-Drug Interactions Involving Rifampicin
Statins have been extensively used worldwide for the treatment of cardiovascular diseases.
