Sublethal renal epithelial cell injury, a key manifestation of drug-induced acute kidney injury (AKI), is characterized by loss of brush border and cellular polarity of proximal tubular cells (PTCs).

Evaluating the Nephrotoxicity of Exemplar Compounds Using a Mechanistic Model of Drug-Induced Acute Kidney Injury
Drug-induced nephrotoxicity is a common source of acute kidney injury (AKI) and a condition that complicates clinical outcomes of vulnerable patients.

Mechanistic Modeling Aids in the Interpretation of Alanine Aminotransferase Elevations Associated with Clinical Ischemic Liver Injury
DILIsym® software can use serial serum alanine aminotransferase (ALT) assessments to predict hepatocyte loss (HL) and corresponding changes in total bilirubin (TBIL) due to...

Physiologically Based Pharmacokinetic Model for Voriconazole and Prediction of its Interactions with Midazolam and Alfentanil
Voriconazole (formerly known as UK-109,496), is a second-generation triazole antifungal agent widely used in the treatment of invasive fungal infections.

Exploring Clinical Relevance of Dissolution Testing by Physiologically Based Biopharmaceutics Modeling (PBBM)
In vitro dissolution testing, if reflective of in vivo drug release/absorption, is considered a surrogate for in vivo drug performance.

Predicting Shrinkage of Individual Parameters in More Complex NLME Models Using Bayesian Fisher Information Matrix
When data are sparse, parameters derived from a non-linear mixed effects model analysis...

Synergy Between Two Mechanisms of Action Contributes to Species Differences in the Liver Safety Profile for PF-04895162
The Purpose is to better understand the mechanisms underlying the apparent species differences, between rat and human, when evaluating the liver safety of compound PF-04895162.

Development of a Physiologically Based Pharmacokinetic (PBPK) Model for Intra-articular Delivery
Understanding local concentrations in intra-articular tissues and fluids such as cartilage, synovial membrane, and synovial fluid are a valuable tool to predict potential...

Efavirenz Physiologically Based Pharmacokinetic Model Development and Validation as a Moderate CYP3A4 Inducer for Drug-Drug Interaction Predictions
Efavirenz is an antiretroviral medication used to treat and prevent HIV/AIDS.

The Effect of the Local Tissue Response on the Pharmacokinetics of Long-Acting Injectable Formulations
Modeling consequences of localized chronic inflammation in tissue on drug diffusion and exposure caused by prolonged therapy with long-acting formulations.

Modeling and Simulation of the Local Tissue Response to the Long-acting Injectable Formulations
Recently, long-acting injectable (LAI) drug formulations have attracted much attention for prolonged drug exposure from weeks to several months.

Adapting a quantitative systems toxicology model of mitochondrial dysfunction in liver to kidney
Kidney, as a major excretory organ, is exposed to high levels of drugs and their metabolites.
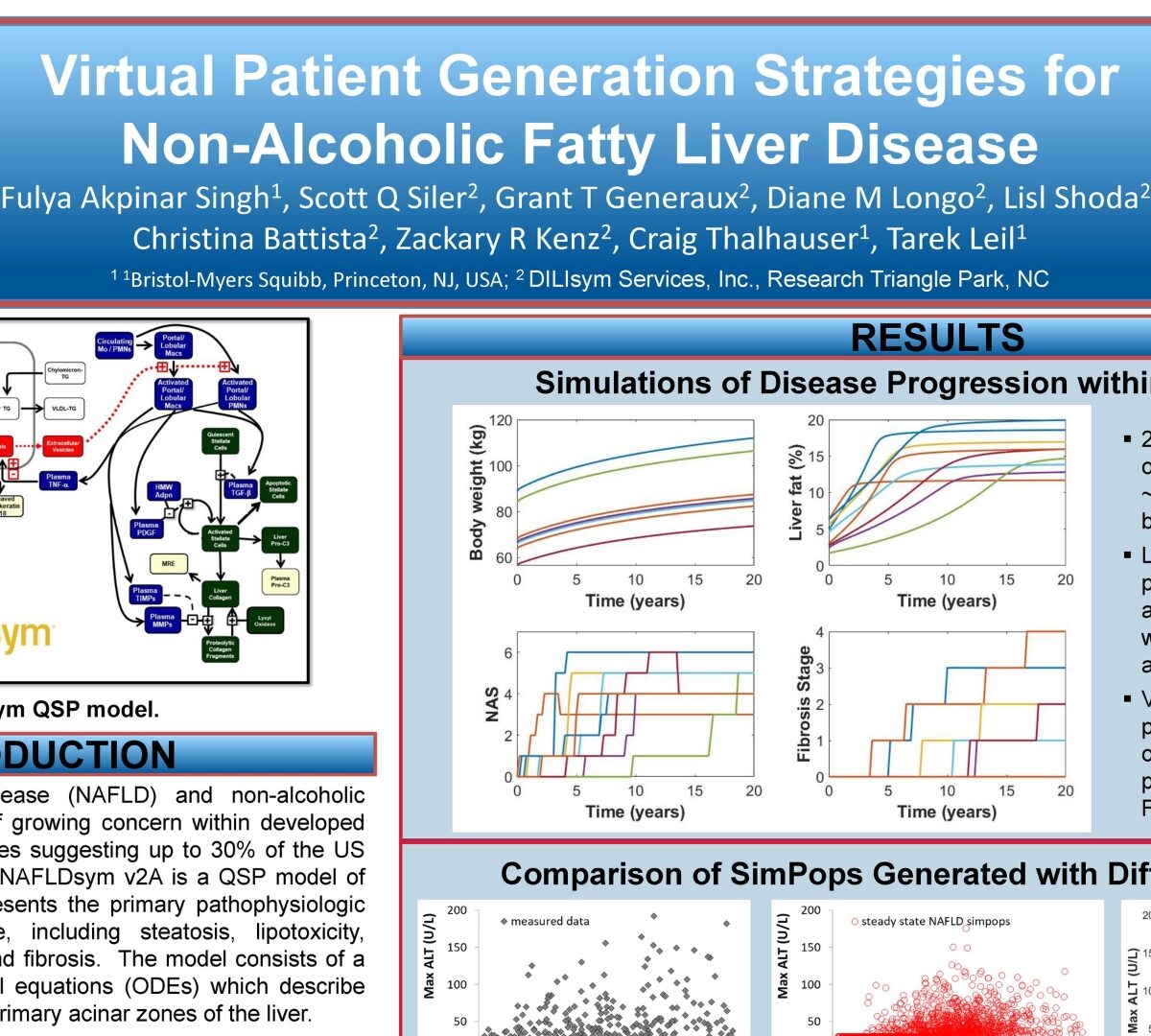
Virtual Patient Generation Strategies for Non-Alcoholic Fatty Liver Disease
Non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) are of growing concern within developed countries, with recent estimates suggesting up to 30% of the US population may be affected.

Mechanistic Analysis of Cisplatin-Induced Acute Kidney Injury Using Quantitative Systems Toxicology Modeling
Acute kidney injury is a common side effect of cisplatin chemotherapy.

The solubility-absorption trade-off in using solubilizers: mechanistic PK simulations of progesterone with explicit cyclodextrin
Cyclodextrins improve solubility of poorly soluble lipophilic drugs due to 1:1 complexation in their nonpolar interior cavity.

Physiologically Based Pharmacokinetic Modeling of Rosuvastatin and Prediction of Transporter-Mediated Drug-Drug Interactions Involving Rifampicin
Statins have been extensively used worldwide for the treatment of cardiovascular diseases.

Development of a Physiologically Based Pharmacokinetic Model for Losartan and Its Active Metabolite E3174 – Ethnic Differences in Pharmacokinetics between Caucasian and Asian Populations
Losartan is a selective, competitive angiotensin II receptor type 1 (AT1) antagonist for hypertension treatment.

Population pharmacokinetic (PopPK) and concentration-QTc analysis of quizartinib in patients (pts) with FLT3-ITD–positive relapsed/refractory (R/R) acute myeloid leukemia (AML)
Fms-related tyrosine kinase 3 (FLT3) is expressed in hematopoietic progenitor cells;
signaling through FLT3 promotes their proliferation and differentiation. FLT3 is mutated
in approximately 30% of patients with AML.

Development of a Direct CD8+ T Cell Activation QSP Model for Ovalbumin in the Context of Liver Injury Advances Groundwork for Mathematical Representation of Idiosyncratic Drug-Induced Liver Injury (iDILI)
Extensive progress has been made in identifying mechanisms for dose-dependent drug-induced liver injury (DILI) and in developing screening assays to reduce its incidence.

Assessing Effects of BHV-0223 40 mg Zydis® Sublingual Formulation and Riluzole 50 mg Oral Tablet on Liver Function Test Parameters Utilizing DILIsym®
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease characterized by the death of motor neurons that leads to progressive muscle weakness and difficulties in speaking, swallowing, and breathing.
