Highly variable disposition after oral ingestion of acyclovir has been reported, although little is known regarding the underlying mechanisms.

Potent and Broad Neutralization of SARS-CoV-2 Variants of Concern (VOCs) including Omicron Sub-lineages BA.1 and BA.2 by Biparatopic Human VH Domains
The emergence of SARS-CoV-2 variants of concern (VOCs) requires the development of next-generation biologics with high neutralization breadth.

Preclinical Pharmacokinetic and Pharmacodynamic Investigation of 5’-Methoxynobiletin from Ageratum conyzoides: In vivo and In silico Approaches
5’-methoxynobiletin (5’-MeONB), a polymethoxyflavone isolated from A. conyzoides, has shown anti-inflammatory property. Nevertheless, the antinociceptive...

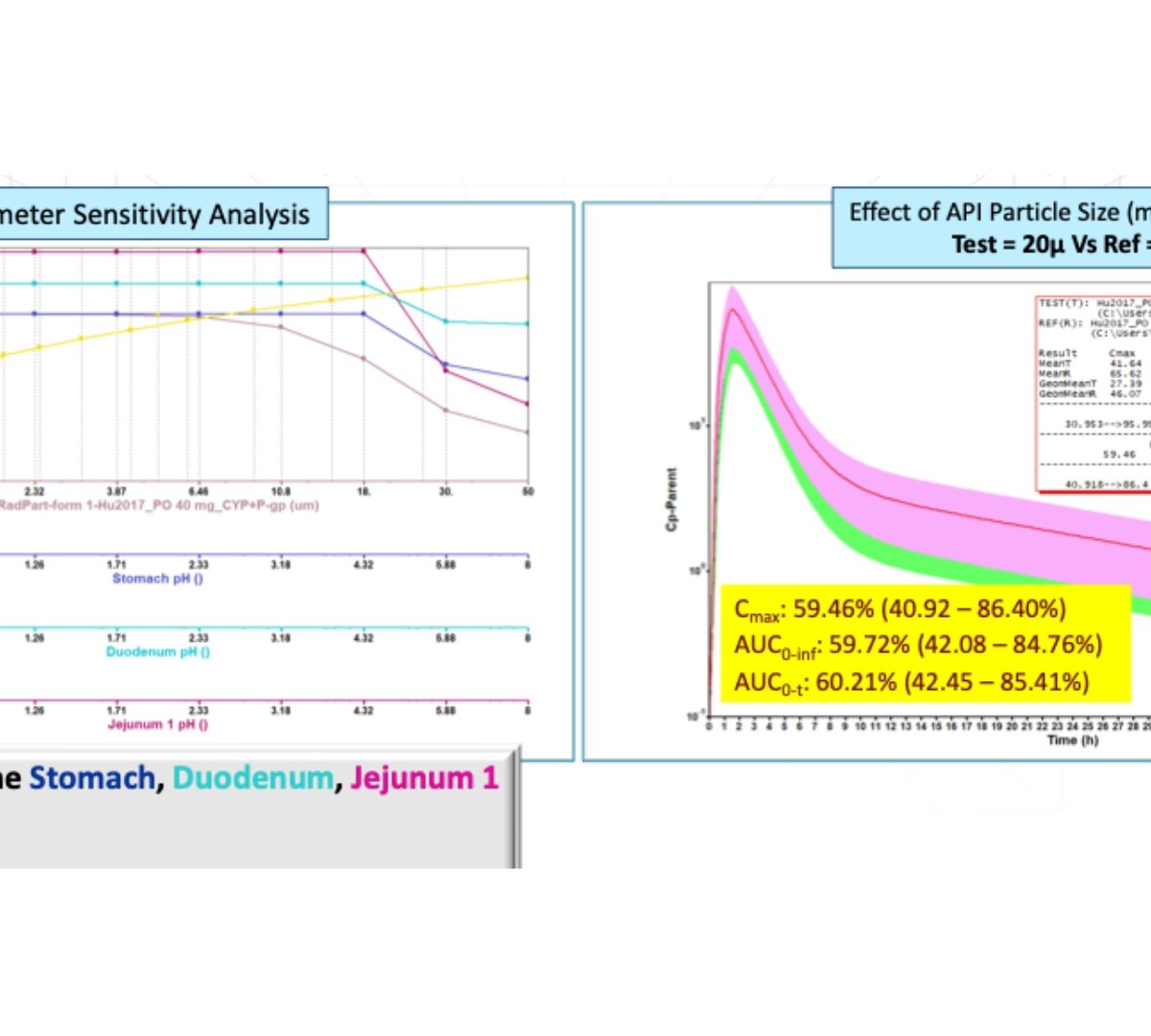
July 2022 GastroPlus Newsletter
Hard to believe that we’re already in the middle of summer.

Identification of a novel immune-inflammatory signature of COVID-19 infections, and evaluation of pharmacokinetics and therapeutic potential of RXn-02, a novel small-molecule derivative of quinolone
Coronavirus disease 2019 (COVID-19) is a global pandemic and respiratory infection that has enormous damage to human lives and economies.

Bioequivalence and Relative Bioavailability Studies to Assess a New Acalabrutinib Formulation That Enables Coadministration With Proton-Pump Inhibitors
Acalabrutinib is a Bruton tyrosine kinase (BTK) inhibitor approved to treat adults with chronic lymphocytic leukemia,small lymphocytic lymphoma, or previously treated mantle cell lymphoma.

Fragment-based drug discovery—the importance of high-quality molecule libraries
Fragment-based drug discovery (FBDD) is now established as a complementary approach to high-throughput screening (HTS).

Simulations Plus Reports Third Quarter Fiscal 2022 Financial Results
Total revenue growth of 17%; Software revenue growth of 16%; Services revenue growth of 19%;
Diluted Earnings Per Share (EPS) growth of 11%;
Board of Directors announces quarterly dividend of $0.06 per share

Understanding Interindividual Variability in the Drug Interaction of a Highly Extracted CYP1A2 Substrate Tizanidine: Application of a Permeability-Limited Multicompartment Liver Model in a Population Based Physiologically Based Pharmacokinetic Framework
Tizanidine, a centrally acting skeletal muscle relaxant, is predominantly metabolized by CYP1A2 and undergoes extensive hepatic first-pass metabolism after oral administration.

First in Human (FIH) PBPK predictions
An overview of what PBPK is will be provided so that everyone starts from the same level.

Simulations Plus Hosts 2nd Annual Pharmacometrics Workshop
Record-setting attendance for the global pharmacometrics community

Using PBPK modeling to assess the impact of diseases on oral drug absorption: case study in HIV-infected patients
Randomized clinical trial is the most effective way to determine whether a cause-and-effect relationship exists between an intervention and a predefined clinical outcome.

Simulation of febuxostat pharmacokinetics in healthy subjects and patients with impaired kidney function using physiologically based pharmacokinetic modeling
Febuxostat is recommended by the American College of Rheumatology Gout Management Guidelines as a first-line therapy for lowering the level of urate in patients with gout.
Data required to build a fit-for-purpose PBPK model: How much is necessary?
PBPK/PBBM model development can be initiated at any stage of product development

Simulations Plus Collaborates with Multinational Pharmaceutical Company to Provide Modeling & Simulation Support for COVID-19 Treatment
Simulations Plus' Cognigen division worked with a major pharmaceutical company to implement modeling and simulation activities to support the development of a candidate COVID-19 therapy.

Simulations Plus Sets Date for Third Quarter Fiscal Year 2022 Earnings Release and Conference Call
Conference call to be on Wednesday, July 6, 2022, at 5:00 PM ET

Impact of Composition and Morphology of Ketoconazole-Loaded Solid Lipid Nanoparticles on Intestinal Permeation and Gastroplus-Based Prediction Studies
Ketoconazole (KTZ) is a potential oral antifungal agent to control systemic and local infections.

From traditional to data-driven medicinal chemistry: A case study
Artificial intelligence (AI) and data science are beginning to impact drug discovery.
