According to the World Health Organization (WHO), COVID-19 has infected more than 5.45 million patients and killed more than 1.25 million people worldwide as of November 10, 2020. To help address this global health crisis, Simulations Plus created the COVID-19 ACT program that prioritizes timely support and finds expedited solutions to address the novel human COVID-19 virus. This transformative approach leverages the capabilities of the entire Simulations Plus family of companies. Cognigen team members Christian Claussen, Kevin Dykstra, and Sebastien Bihorel created a state-of-the-art viral dynamics model describing the course of a severe acute respiratory syndrome coronavirus 2 (SAR-CoV-2) infection.
SARS-CoV-2 Biology
SARS-CoV-2 is the virus that causes the coronavirus disease, also known as COVID-19. SARS-CoV-2 is a beta coronavirus closely related to SARS-CoV that was the infectious agent behind the SARS pandemic appearing in 2002. Characterized by crown-shaped spikes protruding from the spheroidal viral particle, SARS-CoV-2 causes a severe respiratory illness similar to SARS-CoV, though in many cases, even more serious. The virion attaches to the host angiotensin-converting enzyme-2 (ACE2) receptor via interaction with the viral spike protein. The spike is further modified by any one of several host serine proteases, in particular cellular transmembrane protease serine 2 (TMPS2), facilitating viral entry into the cell and initiating the viral replication process. The ACE2 receptor is expressed on oral-nasal mucosal tissue, as well as in the lung alveolar epithelium. Each of these tissues is exposed to open air, providing a locus of exposure to virus-containing aerial droplets or from viral particles found on the skin via facial touching. Loss of taste and smell is a particular early symptom of COVID-19. However, the ACE2 receptor is widely expressed in other organs and tissues, particularly in the heart, kidney, small intestine, and vascular endothelium. In fact, the receptor is expressed in these tissues at higher levels than in the lung. Patients infected with SARS–CoV–2 suffers from progressive, severe respiratory distress. As the disease progresses, they may have effects in other tissues, primarily vascular and renal symptoms, as well as GI symptoms. Some of these may be secondary effects stemming from host inflammatory processes and potential direct sequelae originating from disseminated viremic disease as more tissues expressing ACE2 are exposed to the virus.
The Viral Dynamic Model
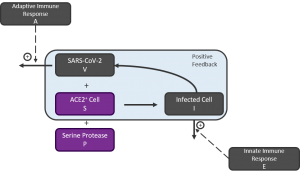
Cognigen Corporation’s highly advanced and interactive viral dynamics model describes a basic SARS-CoV infection mechanism within each tissue. At its core, the mechanistic model includes a positive feedback loop where virus produced by infected cells infects further susceptible cells, resulting in the exponential growth in infected cells and viruses. Describing the model, Dr. Dykstra states: “An uncontrolled infection leads to exponentially increasing viral load, eventually overwhelming the immune system, while an effective immune response, perhaps aided by a therapeutic intervention, results in clearing of the virus.” The initial model describes the body as a whole, while an increasingly granular model would describe individual organs. In a given tissue, susceptibility would be related to the number of ACE2–positive cells and the amount of receptor expressed on the cell surface, and this varies considerably between organs. Additionally, the model expects the rate of infection growth to relate critically to the size of the initial viral inoculum. It is the body’s two-pronged immune response, in the form of the 1) innate and 2) adaptive immune response, that potentially keeps any infection from becoming overwhelming, and this is reflected in the model schematic, provided below, by increased rates of infected cell death and of viral clearance.
Figure 1: Schematic of Basic Viral Kinetic Model
The Impact
This model’s quantitative description of the SARS-CoV-2 replication cycle, interactions with the immune system, and drug effects can be leveraged to explore the potential impact drug candidates might have on various measures of metrics of infection outcome in COVID‐19. Refining the model with emerging data will support more accurate and precise prediction of therapeutic benefit, guiding recommended treatment regimens, and allowing comparisons among development leads. Leveraging the knowledge integrated into the modeling approach can significantly accelerate and de-risk the development of vital therapeutics for this critical disease.
The preliminary version of the model shows the effects of initial viral exposure, immune response, and the timing and dose of an anti-viral treatment on simulated viral load. You can find the interactive version and additional information on the mathematical description and analysis of this viral dynamics model here.
