Bile acids are crucial regulatory molecules that regulate lipid homeostasis and cellular function. However, they are capable of wielding toxic effects when they accumulate in hepatocytes; inhibition of hepatic efflux transporters such as BSEP, MRP3, or MRP4, may result in supraphysiological concentrations of bile acids, leading to hepatotoxicity.
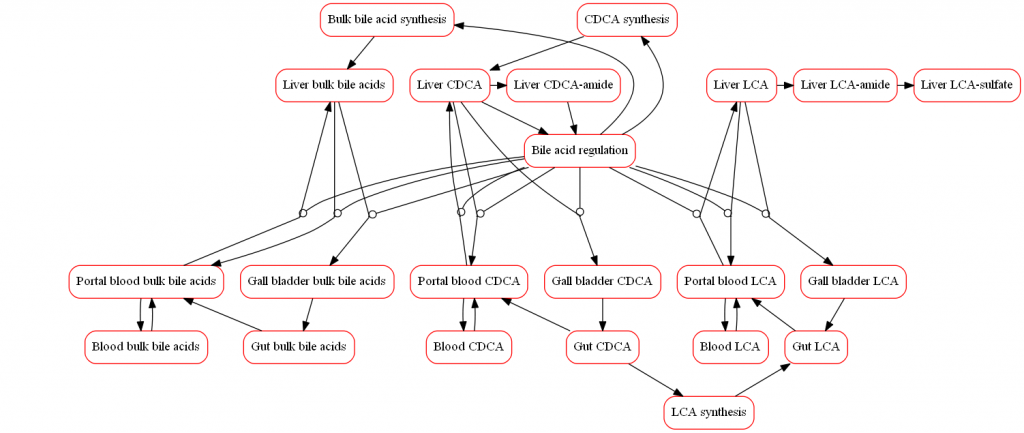
The representation of bile acid biochemistry within the model scope includes the dynamics of specific bile acids implicated in hepatotoxicity. The model incorporates bile acid synthesis, uptake, recirculation, and efflux. Drug-induced changes to bile acid dynamics can increase intracellular bile acids with subsequent hepatotoxicity. Exemplar compounds such as bosentan have been simulated to refine the model and ensure that the modeled representation of bile acid toxicity is consistent with available data.
The mechanism of transporter inhibition can have profound effects on bile acid-mediated hepatotoxicity, and inhibition of any of the hepatic transporters can be simulated as competitive, noncompetitive, or mixed in DILIsym by specifying the inhibition constant (i.e. Ki or IC50) and accompanying alpha value. If data are not available to determine the mode of inhibition, a sensitivity analysis can be performed within the software to see the impact that different modes of inhibition have on hepatotoxicity predictions and determine if further in vitro studies may be warranted to characterize a compound.
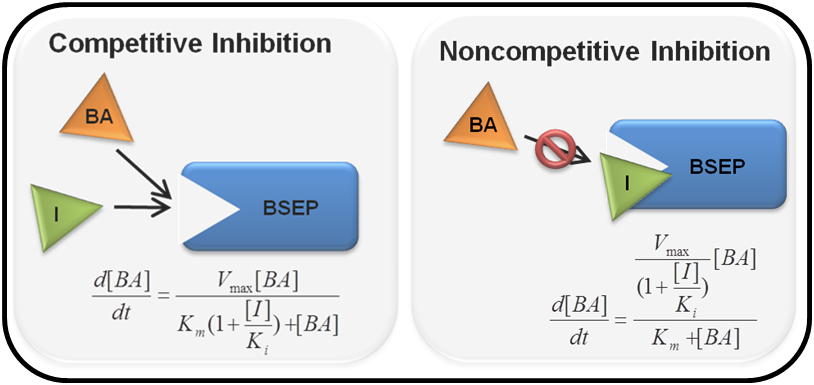
Depiction of different types of inhibition. Competitive inhibition (left) entails an inhibitor using the same binding site as the substrate, decreasing apparent substrate binding affinity. Noncompetitive inhibition (right) occurs when an inhibitor binds to allosteric sites and alters the structure of the protein, allowing substrates to still bind to the active site, but the reaction is prevented.