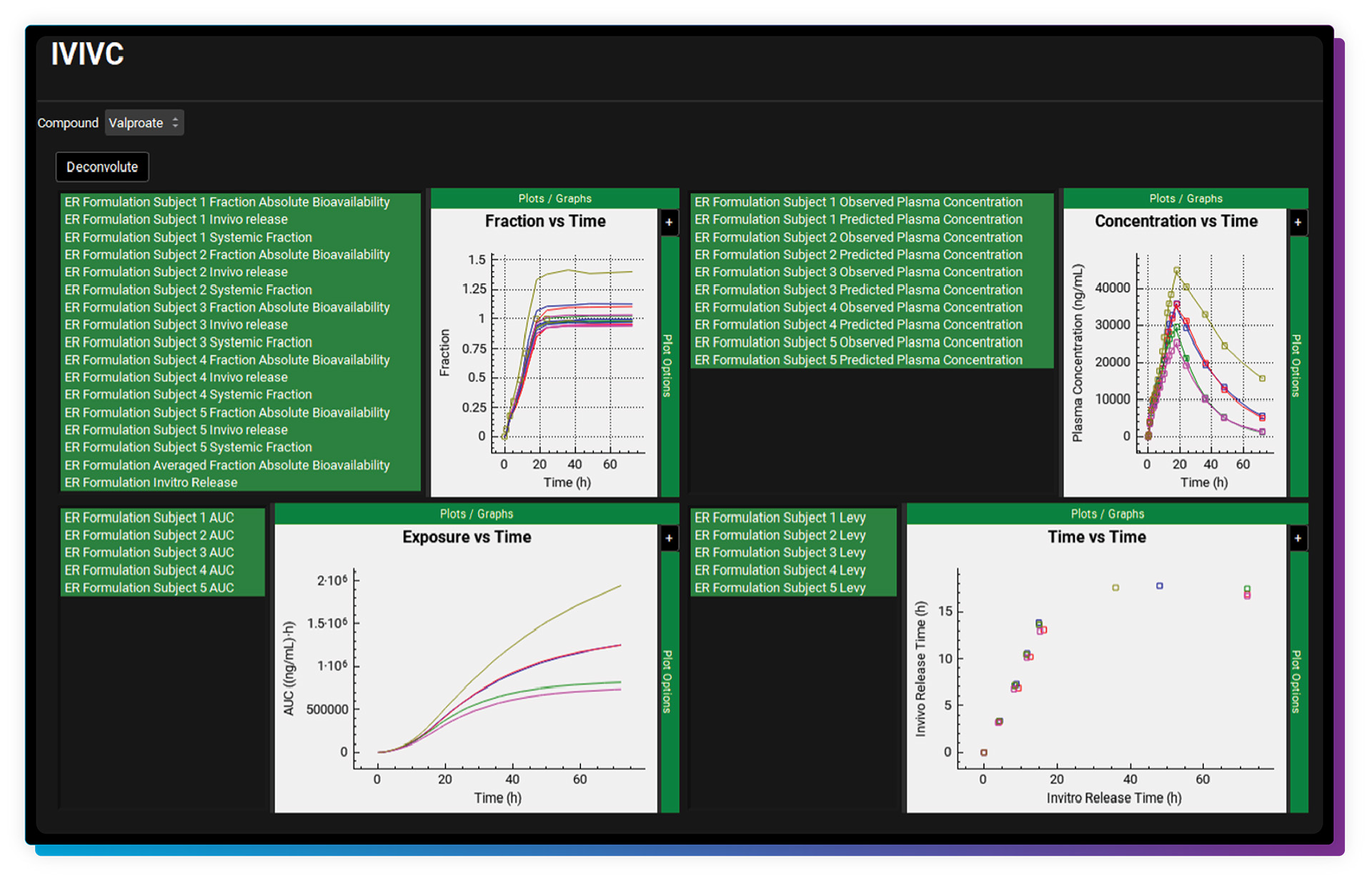
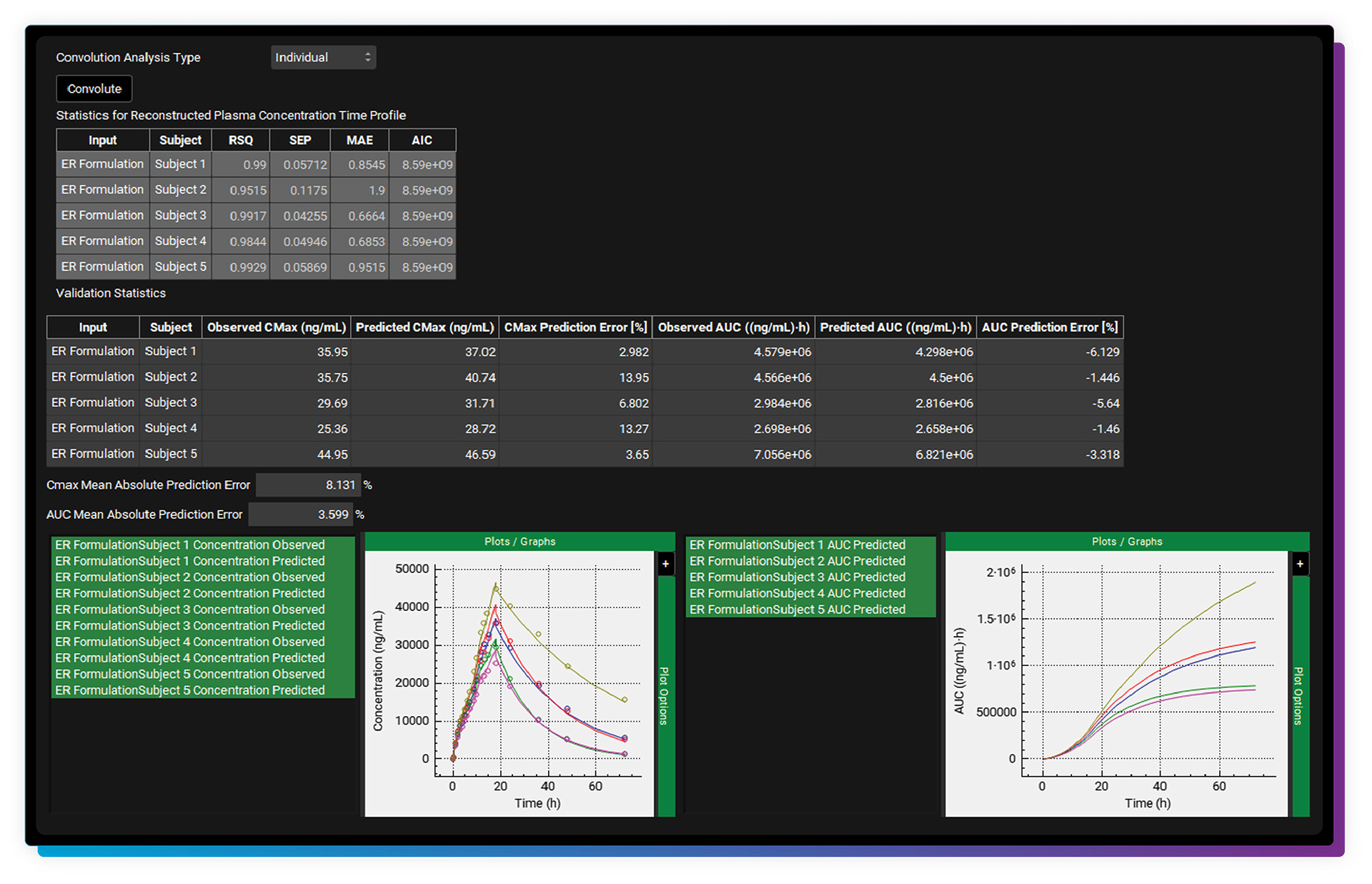
The IVIVCPlus™ Module is an optional add-on module that provides a convenient way to develop mechanistic and/or traditional correlations (IVIVC) between either in vitro release and in vivo release or in vitro release and absolute bioavailability. The formed correlation can then be used to predict plasma concentration-time profiles for formulations with different in vitro release rates or dose strengths to support internal research and regulatory applications (e.g., establish product specifications).
GastroPlus was the first software program to offer “mechanistic deconvolutions”, which deconvolute, or fit, the in vivo dissolution vs. time along the gut lumen using the Weibull function (NEW triple Weibull function options!). An advantage to using the mechanistic deconvolution method is that it can be linked to a PBPK & PBBM model (through the PBPKPlus™ Module). We are pleased to validate the mechanistic deconvolution method through a 5-year Research Collaboration Agreement with the U.S. FDA.