Population exposure‑response analysis was undertaken to describe the relationship of drug concentrations to measures of clinical efficacy and safety in patients with schizophrenia or bipolar mania.

The Steady-State Pharmacokinetic Profile Across a Range of Patient Body Weight Categories Supports Weight-Based Dosing for Intravenous Reslizumab
Reslizumab is an IgG4 kappa humanized monoclonal antibody that targets interleukin-5, leading to marked reductions in airway eosinophil levels, and is indicated as an ‘add-on’ maintenance treatment for adult...

Physiologically Based Pharmacokinetic (PBPK) Model to Describe Absorption and Disposition of Inhaled Capreomycin
The current work describes the simulation of capreomycin pharmacokinetics (PK) after IV and pulmonary administration. Capreomycin is an antibiotic used to treat tuberculosis. It is eliminated mainly by...

Use of Pharmacokinetic-Pharmacodynamic Modeling and Simulations to Predict Efficacy Outcomes with Eslicarbazepine Acetate 800 mg Once Daily as Monotherapy
Eslicarbazepine acetate (ESL) is a once-daily (QD) oral antiepileptic drug (AED), approved as adjunctive treatment for partial-onset seizures (POS) in the USA, Europe, and Canada, and as monotherapy for...

Integrating Predictions from Complementary Cytochrome P450 (CYP) Models
Many structure-activity classification models have been published for predicting whether a given compound is likely to inhibit and/or be subject to metabolism by a given cytochrome P450 (CYP) isoform, and...

QSAR-based Prediction of Ames Mutagenicity for ICH M7 Submissions
The “Ames test”, originally developed by Bruce Ames and his group, is a way to measure the mutagenic potential of chemicals.1 It uses strains of Salmonella typhimurium and Escherichia coli as an...
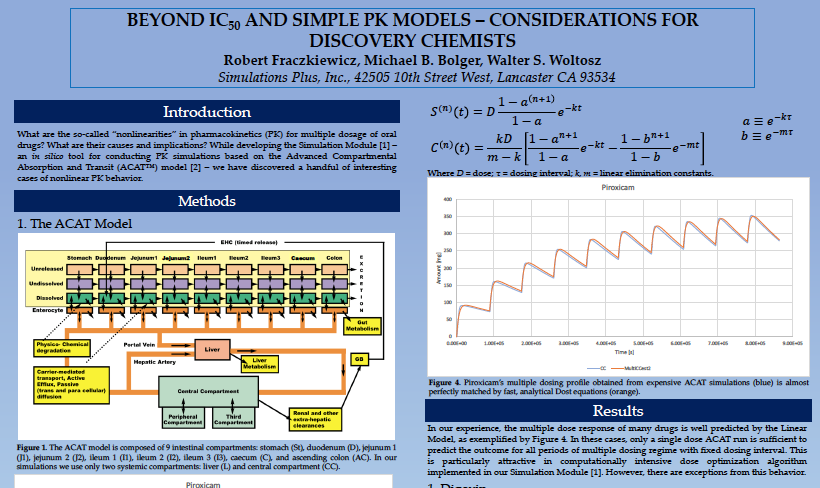
Beyond IC50 and simple PK models – Considerations for discovery chemists
While developing the Simulation Module [1] – an in silico tool for conducting PK simulations based on the Advanced Compartmental Absorption and Transit (ACAT™) model [2] – we have discovered a handful of...

Mechanistic Modeling Predicts Drug-Induced HyperbilirubinemiaThat Involves Inhibition Of Enzymes And Transporters
Elevated serum ALT and bilirubin indicates high risk of fatal drug-induced liver injury. However, drugs also can increase serum bilirubin in the absence of hepatic injury by inhibiting enzymes and/or transporters.

in silico Prediction of Oral Bioavailability
Oral bioavailability (F%) is an important pharmacokinetic property that can determine the fate of a compound in clinical trials. Predicting F% directly from the 2D structure of the molecule prior to first…

Using Physiologically Based Pharmacokinetic Modeling for in vitro – in vivo Extrapolation to Predict Chemical Exposure
Mechanistic absorption and physiologically based pharmacokinetic (MA/ PBPK) models are useful tools in risk assessment. These models incorporate complex processes related to a compound’s disposition...

QSAR Modeling Independent of Input Tautomers
The quality and predictivity of QSAR models used in drug design and development often depend on the tautomeric and valence structures used to represent the molecules of interest. This is because the...

in silico Modeling of Aryl Hydrocarbon Receptor (AhR) Activation
Sustained activation of AhR is the molecular initiating event (MIE) in several adverse outcome pathways (AOPs)1: Lethality to fish and bird embryos, Causing uroporphyria in birds, Producing liver tumors in...

Mechanistic Absorption and Physiologically Based Pharmacokinetic Modeling of Itraconazole and Its Application for Drug-Drug Interaction with Midazolam in Adult Populations
Itraconazole (ITZ) is a BCS Class II triazole antifungal (Sporanox; Janssen Pharmaceutica, Titusville, NJ). It is a substrate and potent inhibitor of CYP3A4. The primary metabolite hydroxy-itraconazole...

Application of PBPK Modeling to Predict Monoclonal Antibody Disposition after Intravenous and Subcutaneous Administration in Rats and Humans
Therapeutic monoclonal antibodies (mAbs) represent a growing segment of the development pipeline in the pharmaceutical industry. Physiologically based pharmacokinetic (PBPK) modeling has been extensively...

Physiologically Based Pharmacokinetic (PBPK) Model for Prediction of Vancomycin Pharmacokinetics in Children
Ethical considerations prevent extensive clinical trials in pediatric populations; however, with the use of PBPK modeling, in vivo data from adults can be used to explore the mechanisms of drug...

Single-dose, Multiple-dose, and Population Pharmacokinetics of SUN13837 Injection, a Basic Fibroblast Growth Factor Mimic in Healthy Subjects
SUN13837, a novel small molecule in development for the treatment of acute spinal cord injury and stroke with biological activities similar to basic fibroblast growth factor (bFGF) promotes cellular…

Physiologically Based Pharmacokinetic Modeling of Rosuvastatin and Prediction of Transporter-Mediated Drug-Drug Interactions Involving Gemfibrozil
Rosuvastatin (Crestor®) is a commonly prescribed lipid-lowering agent from the statin drug class for the treatment of primary hyperlipidemia and hypertriglyceridemia. It may be coprescribed with...

Quantitative Approach to Predicting Human Pharmacokinetics of Monoclonal Antibody (DSP-mAbX) from Preclinical Data
DSP-mAbX is a full human monoclonal antibody targeted to a cell surface antigen. The cross-reactivity of DSP-mAbX to the target antigen in human and monkey has already been confirmed. Here, a quantitative...

Systemization of Logistic Regression Analysis for Pharmacometric Applications
Efficacy and safety data are oftentimes collected as binary (yes/no) data in clinical trials during drug development. The implementation and growing use of CDISC standards in data collection and...

Mechanistic Modeling With Dilisym® Predicts Dose-Dependent Clinical Hepatotoxicity Of Amg 009 That Involves Bile Acid Transporter Inhibition
To predict the clinical hepatotoxicity of AMG 009 and species differences in AMG 009-mediated hepatotoxicity using DILIsym®, a mechanistic model of drug-induced liver injury
