The first algorithm ever developed for processing by machine was designed by Ada Lovelace, a pioneering mathematician in the 19th century.

Evaluation of Antibacterial, Cytotoxicity, and Apoptosis Activity of Novel Chromene-Sulfonamide Hybrids Synthesized Under Solvent-Free Conditions and 3D-QSAR Modeling Studies
In this study, eleven novel chromene sulfonamide hybrids were synthesized by a convenient method in accordance with green chemistry.

Systematic Development of Hot Melt Extrusion-Based Amorphous Solid Dispersion: Integrating Quality by Design and In Silico Modeling
The study aimed to develop and optimize apremilast (APST) solid dispersion formulations using copovidone (Kollidon VA64) as the carrier and vitamin E TPGS as the surfactant to enhance solubility and dissolution, and to utilize in silico Physiologically Based Biopharmaceutics Modeling (PBBM) in GastroPlus to simulate the in vivo behaviour of the optimized formulation, predicting its potential for enhancing oral bioavailability.

In Silico Technologies A Strategic Imperative for Accelerating Breakthroughs and Market Leadership for FDA-regulated Products
The 21st century technology revolution has the power to reshape the way organizations manage, process, and harness data, drive innovation, and unlock new possibilities for consumers and patients across the globe

Evaluation of Pharmacokinetics of a BCS Class III Drug with Two Different Study Designs: Tenofovir Alafenamide Monofumarate Film-coated Tablet
Tenofovir alafenamide (TAF) is a BCS Class III compound and an oral pro-drug of Tenofovir (TFV) with limited oral bioavailability.

Applying Physiologically Based Pharmacokinetic Modeling to Interpret Carbamazepine’s Nonlinear Pharmacokinetics and Its Induction Potential on Cytochrome P450 3A4 and Cytochrome P450 2C9 Enzymes
Carbamazepine (CBZ) is commonly prescribed for epilepsy and frequently used in polypharmacy.

Building Patient Trust with Next-Generation Clinical Trial Training
Patient trust is critical to ensuring adequate enrollment and retention of clinical trial participants.

Conventional vs Mechanistic IVIVC: A Comparative Study in Establishing Dissolution Safe Space for Extended Release Formulations
The use of in vitro-in vivo correlation (IVIVC) for extended release oral dosage forms is an important technique that can avoid potential clinical studies.

Construction of a physiologically based pharmacokinetic model of paclobutrazol and exposure estimation in the human body
Paclobutrazol (PBZ) is a plant growth regulator that can delay plant growth and improve plant resistance and yield. Although it has been widely used in the growth of medicinal plants, human beings may take it by taking traditional Chinese medicine.

Evaluating gender effect in the generic bioequivalence studies by physiologically based pharmacokinetic modeling – A case study of dextromethorphan modified release tablets
The United States Food and Drug Administration guidelines for the bioequivalence (BE) testing of the generic drug products suggests that there should be an equal proportion of male and female population in the BE study.

Simulations Plus to Participate in 21st Annual Craig-Hallum Institutional Investor Conference
Simulations Plus to Participate in 21st Annual Craig-Hallum Institutional Investor Conference
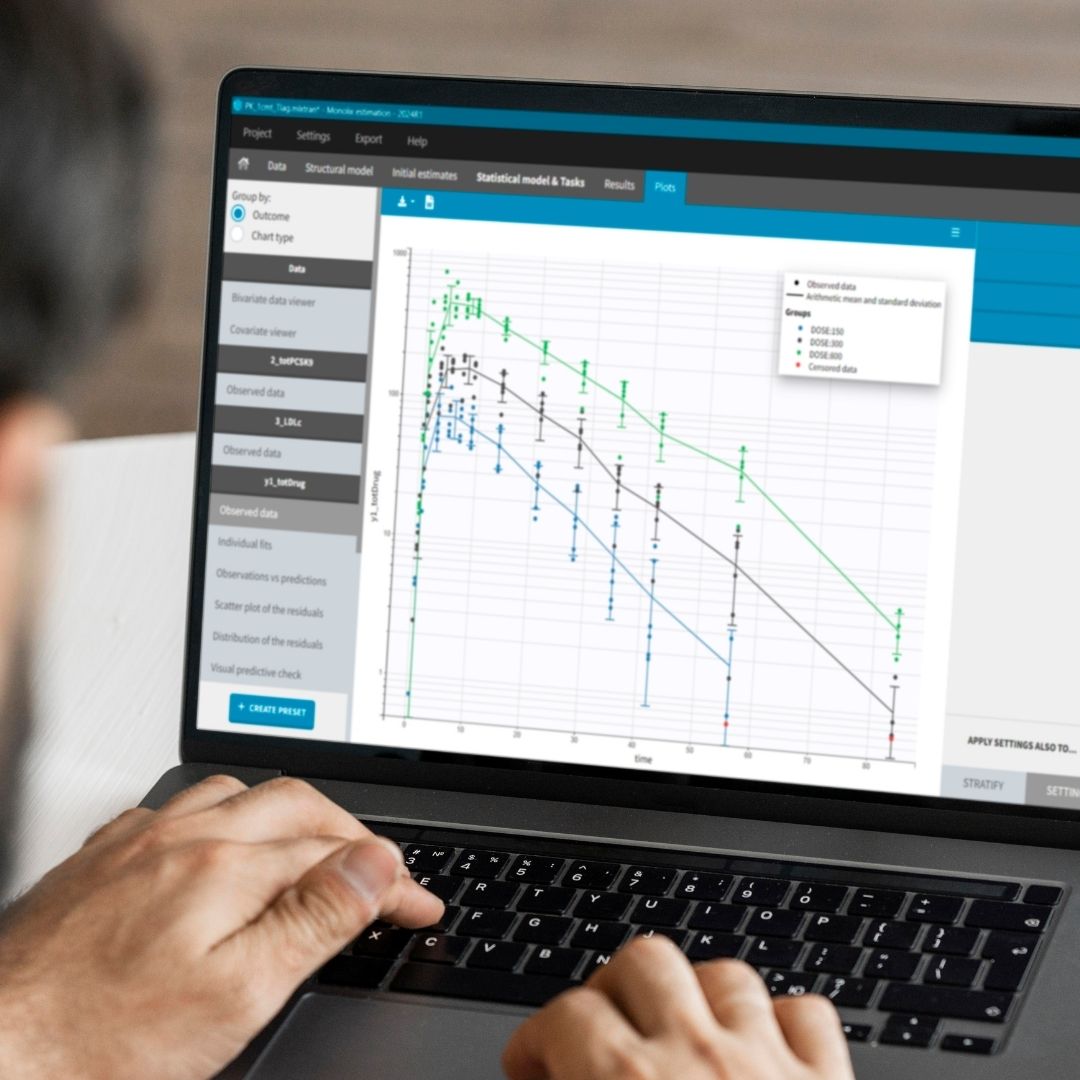
Monolix Comparison Flyer
Your software should make it easier to conduct non-compartmental, bioequivalence and compartmental analysis and use that data to make the right decisions for your program.

Regulatory Strategies Flyer
Get to Market Faster with Robust Regulatory Strategies

Clinical Pharmacology & Pharmacometrics Software and Services Flyer
Supporting Dose Selection and Justification
Cheminformatics Software & Services Flyer
Understand Your Compound

PBPK Software and Services Flyer
Optimize Decision Making Throughout R&D with PBPK Predictions
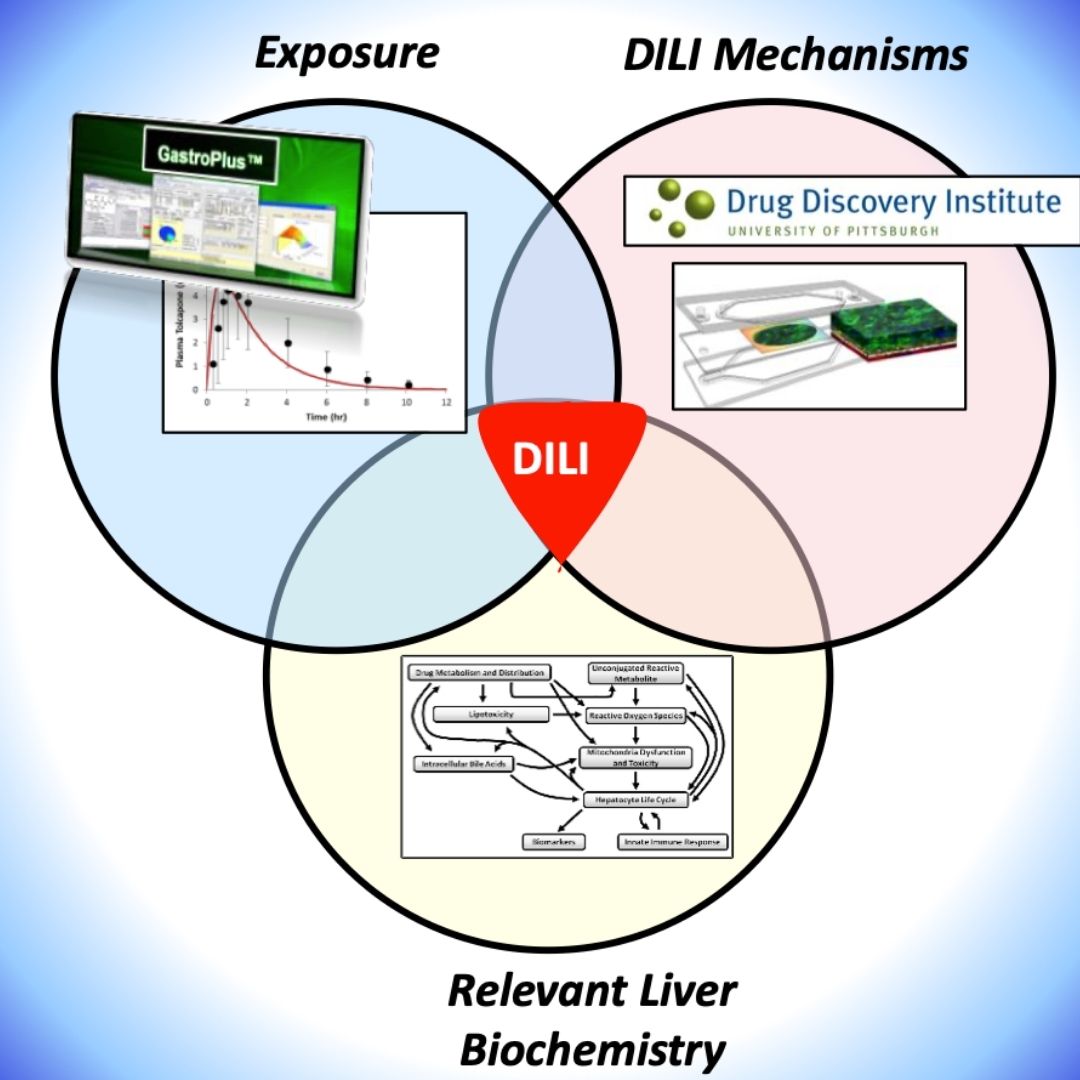
Integrating Human Biomimetic Liver Microphysiology System with Quantitative Systems Toxicology Modeling to Predict DILI
Integrating Human Biomimetic Liver Microphysiology System with Quantitative Systems Toxicology Modeling to Predict DILI

Demystifying GPX™ Installation: Expert Insights and Practical Tips
Get ready to turbocharge your scientific team’s PBBM PBPK modeling technology with the latest version of GastroPlus®!

GPX™ The Next Level PBPK Platform
It’s here—the latest version of GastroPlus®, GPX. And this is your chance to see it in action.