Quantitative Systems Pharmacology (QSP)
Multiple Myeloma Model


Quantitative Systems Pharmacology (QSP)
Multiple Myeloma Model

Quantitative Systems Pharmacology (QSP)
Melanoma Model

Quantitative Systems Pharmacology (QSP)
QSP Diffuse Large B Cell Lymphoma (DLBCL) Model
Quantitative Systems Pharmacology (QSP)
Acute Myeloid Leukemia (AML) and Myelodysplastic Syndrome (MDS) Models
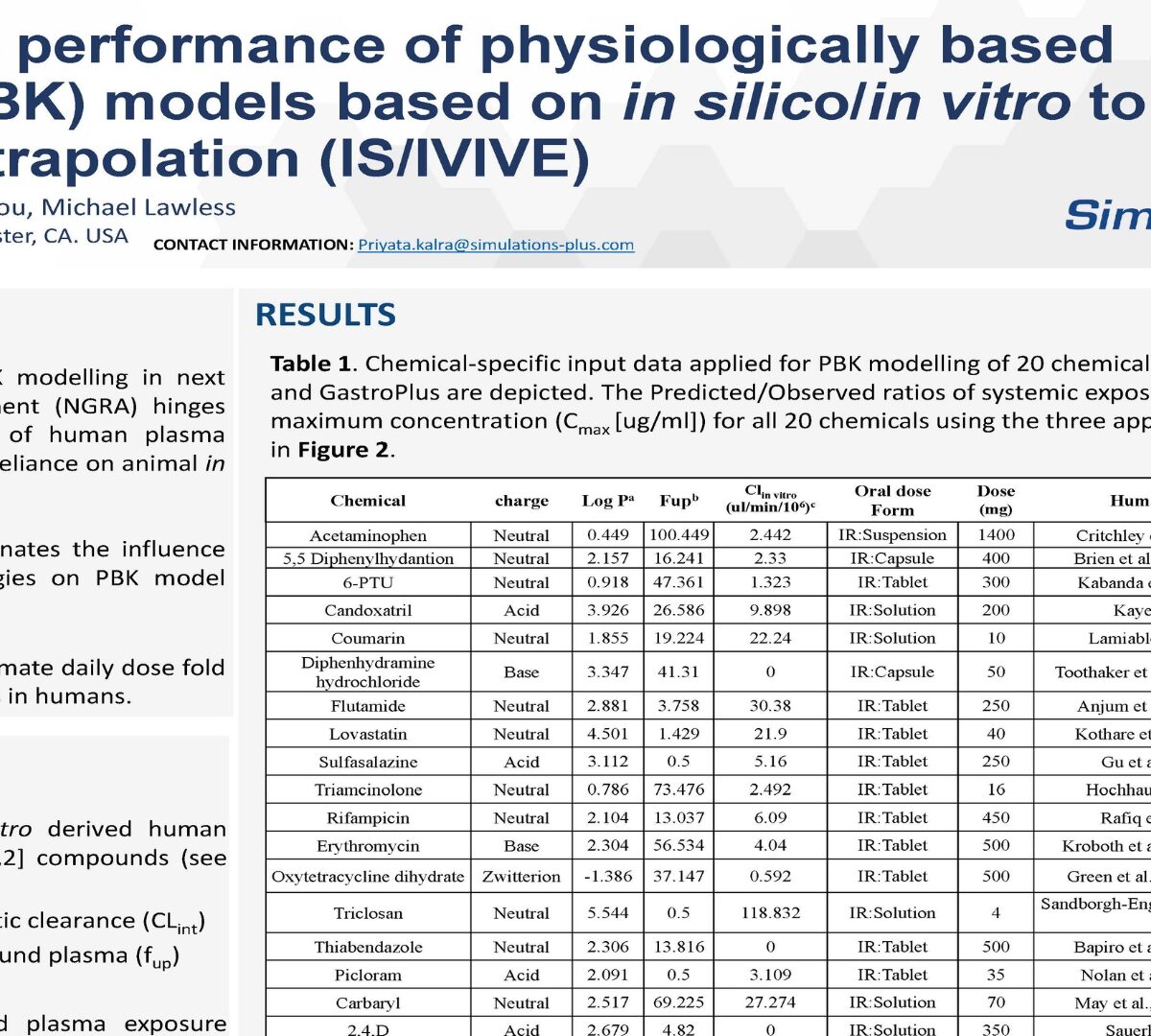
The applicability of PBK modelling in next generation risk assessment ( hinges on accurate prediction of human plasma concentrations without reliance on animal in vivo kinetics data.

Immersive activities helped attendees globally learn and apply best practices for PBPK modeling

Increasing the probability of success in clinical trials
![The anti-infective crotalicidin peptide analog RhoB-Ctn[1–9] is harmless to bovine oocytes and able to induce parthenogenesis in vitro](https://www.simulations-plus.com/wp-content/themes/simulations-plus/library/dist/img/default_square-large.jpg)
Crotalicidin is a cathelicidin-related anti-infective (antimicrobial) peptide expressed in the venom glands of the South American rattlesnake Crotalus durissus terrificus.
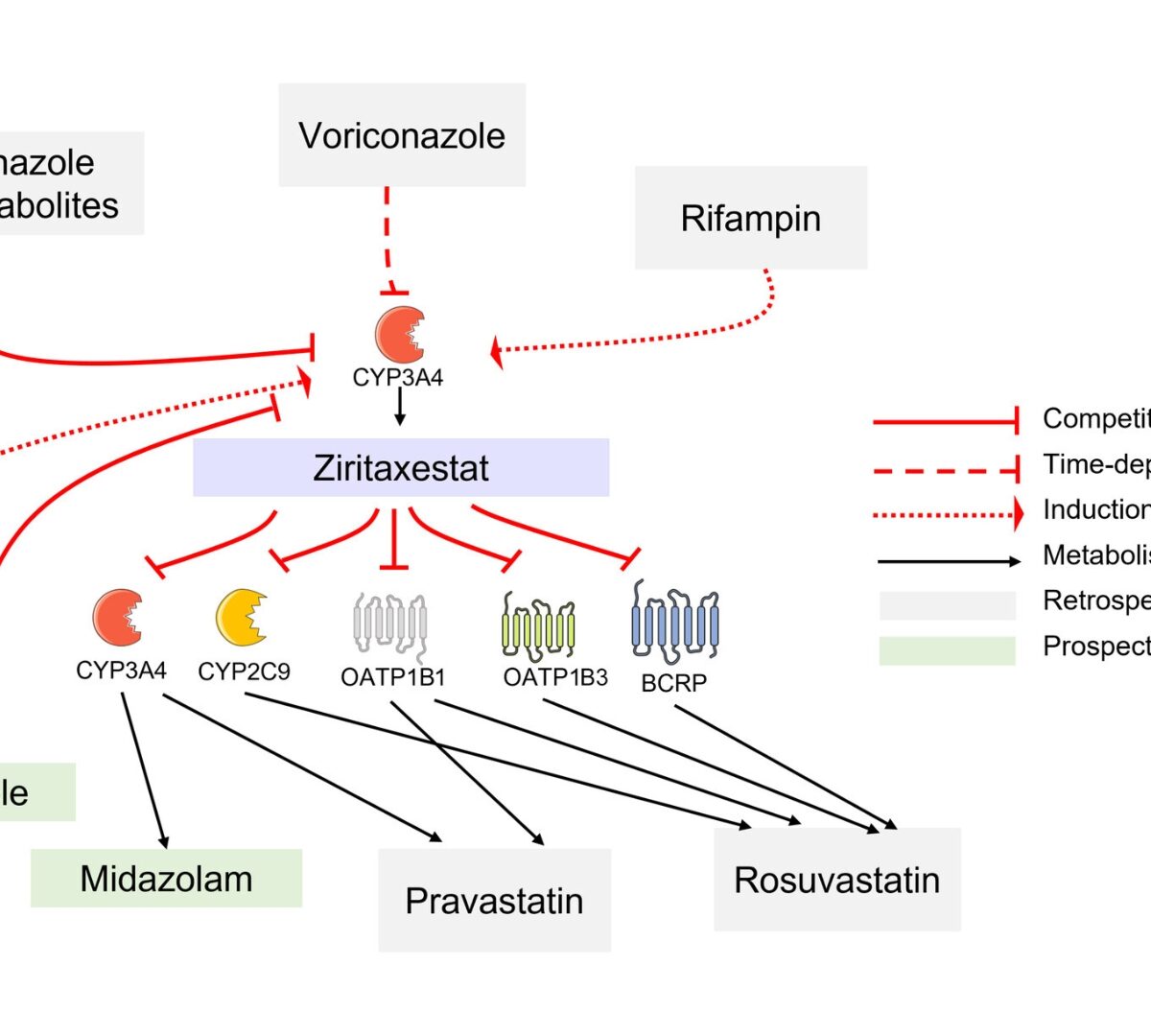
Ziritaxestat, an autotaxin inhibitor, was under development for the treatment of idiopathic pulmonary fibrosis.
Encorafenib, a potent BRAF kinase inhibitor undergoes significant metabolism by CYP3A4 (83%) and CYP2C19 (16%) and also a substrate of P-glycoprotein (P-gp).

The P2Y12 receptor antagonist selatogrel is being developed for subcutaneous self-administration with a ready-to-use autoinjector at the onset of acute myocardial infarction (AMI)symptoms.
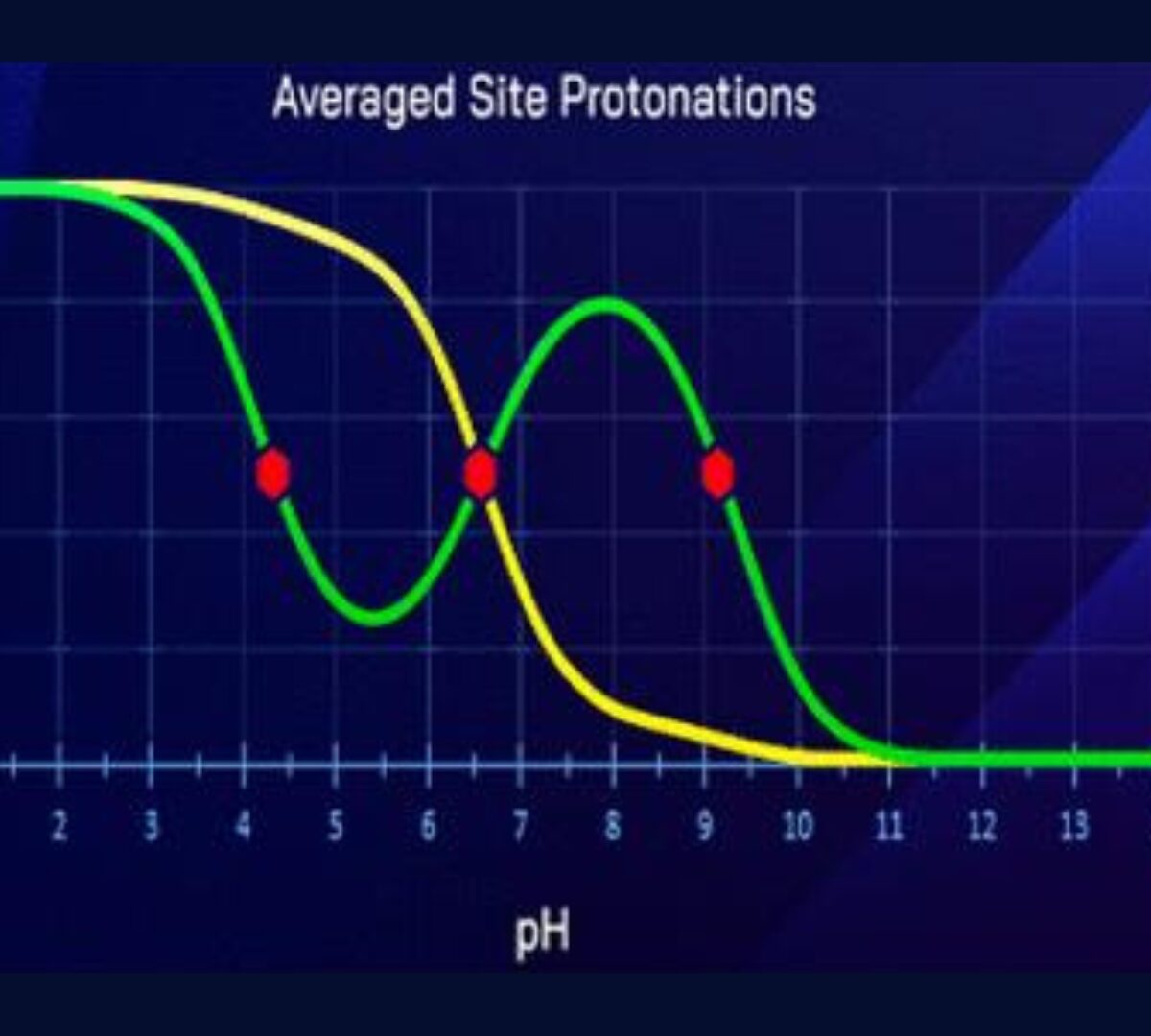
We discovered an anomalous basic dissociation in certain multiprotic compounds.
Dabigatran is the first of four direct-acting oral anticoagulants approved to prevent stroke in adult patients with atrial fibrillation using a fixed two-dose scheme compared with warfarin dosing adjusted to...
Encorafenib, a potent BRAF kinase inhibitor gets significantly metabolised by CYP3A4 (83%) and CYP2C19 (16%) and is a substrate for P-glycoprotein...

Pyronaridine-artesunate was recently strongly recommended in the 2022 update of the WHO Guidelines for the Treatment of Malaria, becoming the...
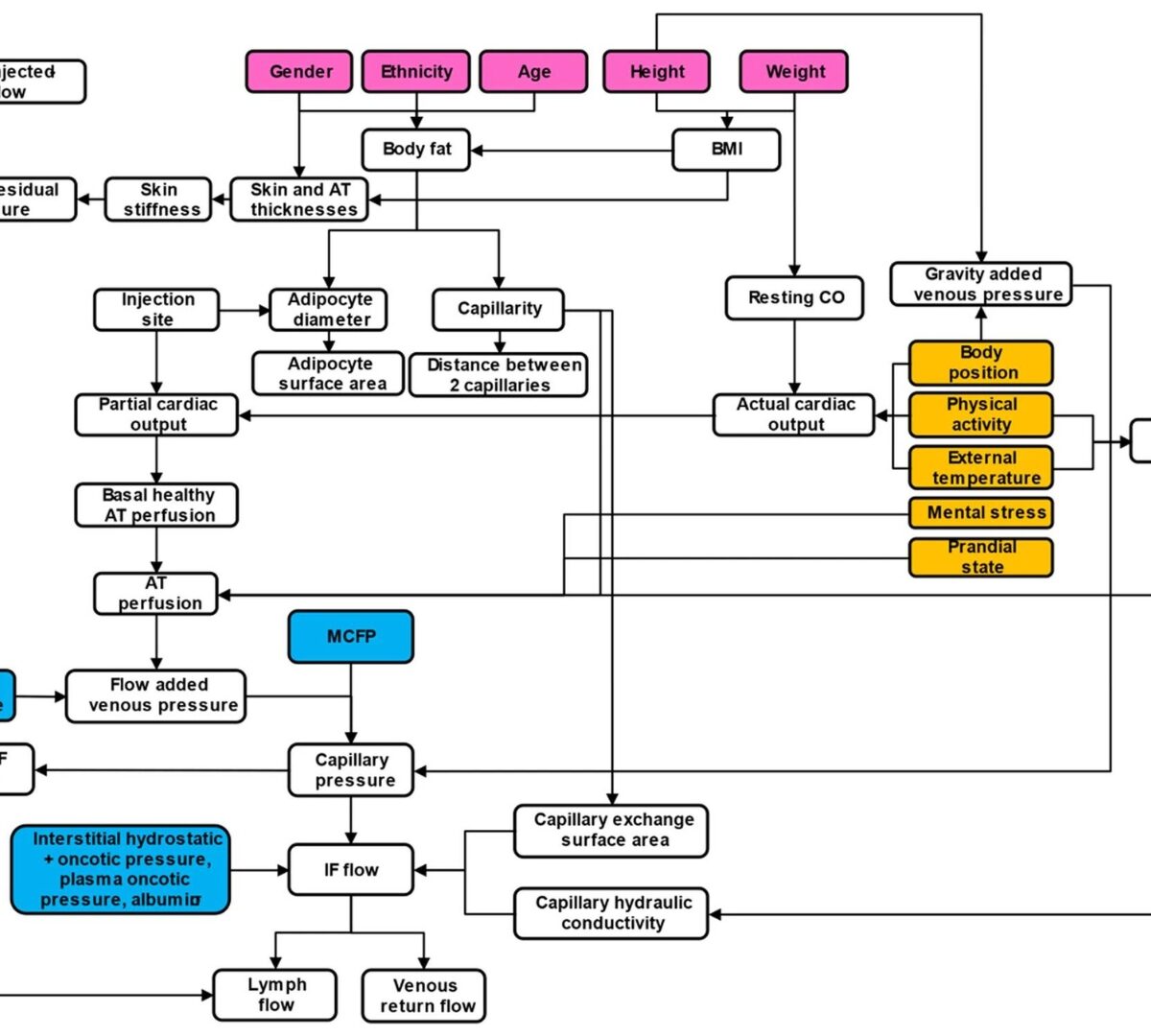
To construct a detailed mechanistic and physiologically based biopharmaceutics model capable of predicting 1) device-formulation-tissue interaction during the injection process and 2) binding, degradation, local distribution, diffusion, and drug absorption, following subcutaneous injection.

Dr. Lukacova’s outstanding contributions to pharmaceutical research and innovation to be recognized with seven others at AAPS PharmSci 360
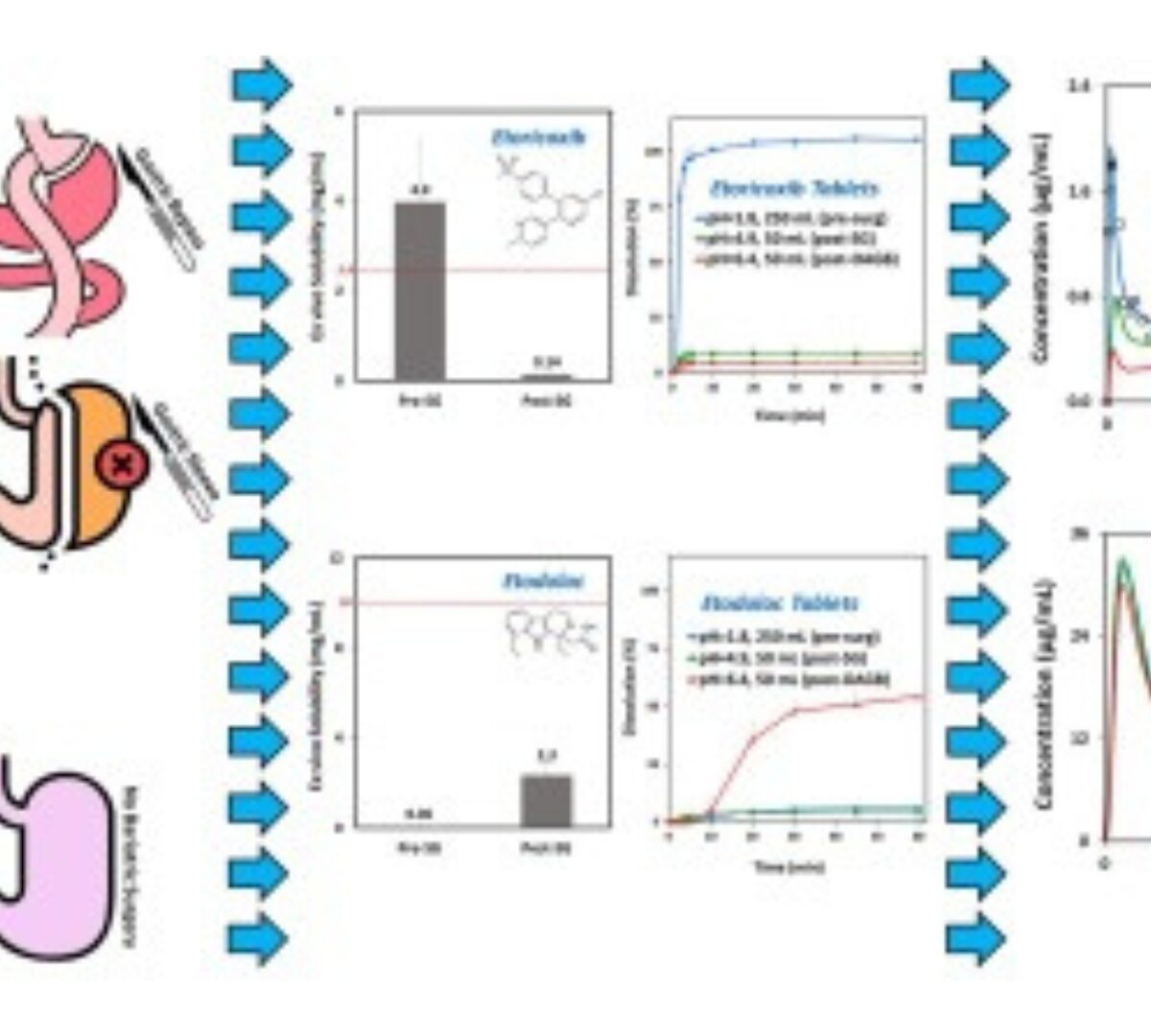
Anatomical/physiological gastrointestinal changes after bariatric surgery may influence the fate of orally administered drugs.

Reliable, experimentally determined partition coefficient P (logP) for most drugs are often unavailable in the literature. Many values are from in silico predictions and may not accurately reflect drug lipophilicity.

Introduction to drug development
Introduction to PBPK modeling
Introduction to QSP/QST modeling