Botanicals have broad use as traditional medicines, natural health products, and dietary supplements around the world.
Your #1 Ranked Team for Exposure and Safety Solutions!
Do you think modeling and simulation might help?

Modeling of Cyclosporine A-Induced Acute Kidney Injury with RENAsym®
Cyclosporine A (CsA) is an immunosuppressant commonly used to prevent organ rejection and can be used to treat other diseases such as...

Mechanistic modeling of biologics-induced liver injury (BILI) predicts hepatotoxicity of Tocilizumab through both on- and off-target effects
Biologics address a range of unmet medical needs. However, there are
increasing numbers of BILI cases which slow therapeutic development...

Simulating Multidrug Resistance Protein 3 (MDR3) Inhibition-Mediated Cholestatic Liver Injury Using DILIsym X, a Quantitative Systems Toxicology (QST) Modeling Platform
Inhibition of efflux transporters located on the canalicular membrane of hepatocytes is an important mechanism of cholestatic liver injury, which is...

Quantitative Systems Toxicology (QST) Modeling of Cimaglermin Alfa (GGF2) Hepatotoxicity Shows the Potential of BIOLOGXsym to Predict Biologics-Induced Liver Injury (BILI)
Biologics continue to address various unmet medical needs, but the occurrence of BILI can terminate clinical...

Modeling of Indinavir-Induced Crystal Nephropathy in RENAsym®
Indinavir, a protease inhibitor used to treat patients with HIV/AIDS, is known to induce crystal nephropathy. This is...

Evaluating Nephrotoxicity of Cisplatin in Rats with RENAsym, a Mechanistic Model of Drug-Induced Acute Kidney Injury
Acute kidney injury (AKI) is often initiated by cellular toxicity of proximal tubule epithelial cells (PTEC) when...

PBK modelling of topical application and characterisation of the uncertainty of Cmax estimate: A case study approach
Combined with in vitro bioactivity data, physiologically based kinetic (PBK) models has increasing applications in next generation risk assessment for animal-free safety decision...

Simulations Plus Hosts the 2022 Model-Informed Drug Development (MIDD+) Scientific Conference
Attendees from 57 Countries Spend Two Days Focused on Advancing Model-Informed Drug Development
Building a Machine Learning Model for Tautomer Prediction
Many drug molecules exhibit tautomerism

Simulations Plus Sets Date for Second Quarter Fiscal Year 2022 Earnings Release and Conference Call
Conference Call to be on Wednesday, April 6, 2022, at 5:00 PM ET

Furthering the clinical development of navicixizumab in advanced epithelial ovarian cancer patients with a population pharmacokinetic model and exploratory exposure-safety-efficacy response analyses
Navicixizumab is a first-in-class, bispecific, anti-angiogenic antibody to vascular endothelial growth factor (VEGF) and
AI-driven Lead Discovery
Computer based de novo drug design background

Identification and characterization of urapidil stress degradation products by LC-Q-TOF-MS and NMR: Toxicity prediction of degradation products
Urapidil, an antihypertensive drug is subjected to various stress conditions (acidic, basic, neutral, oxidative and photolytic) as per ICH guidelines.

Using artificial intelligence to design BACE1 inhibitors
β-secretase 1 (BACE1) is an enzyme involved in production of amyloid-βpeptides, which are involved in the pathology of Alzheimer’s disease.

The evaluation of drug-plasma protein binding interaction on immobilized human serum albumin stationary phase, aided by different computational approaches
The drug-human serum albumin binding interaction was evaluated on a stationary phase immobilized with human serum albumin using a mixture of phosphate buffer (pH 7.0) and acetonitrile modifier as mobile phase.

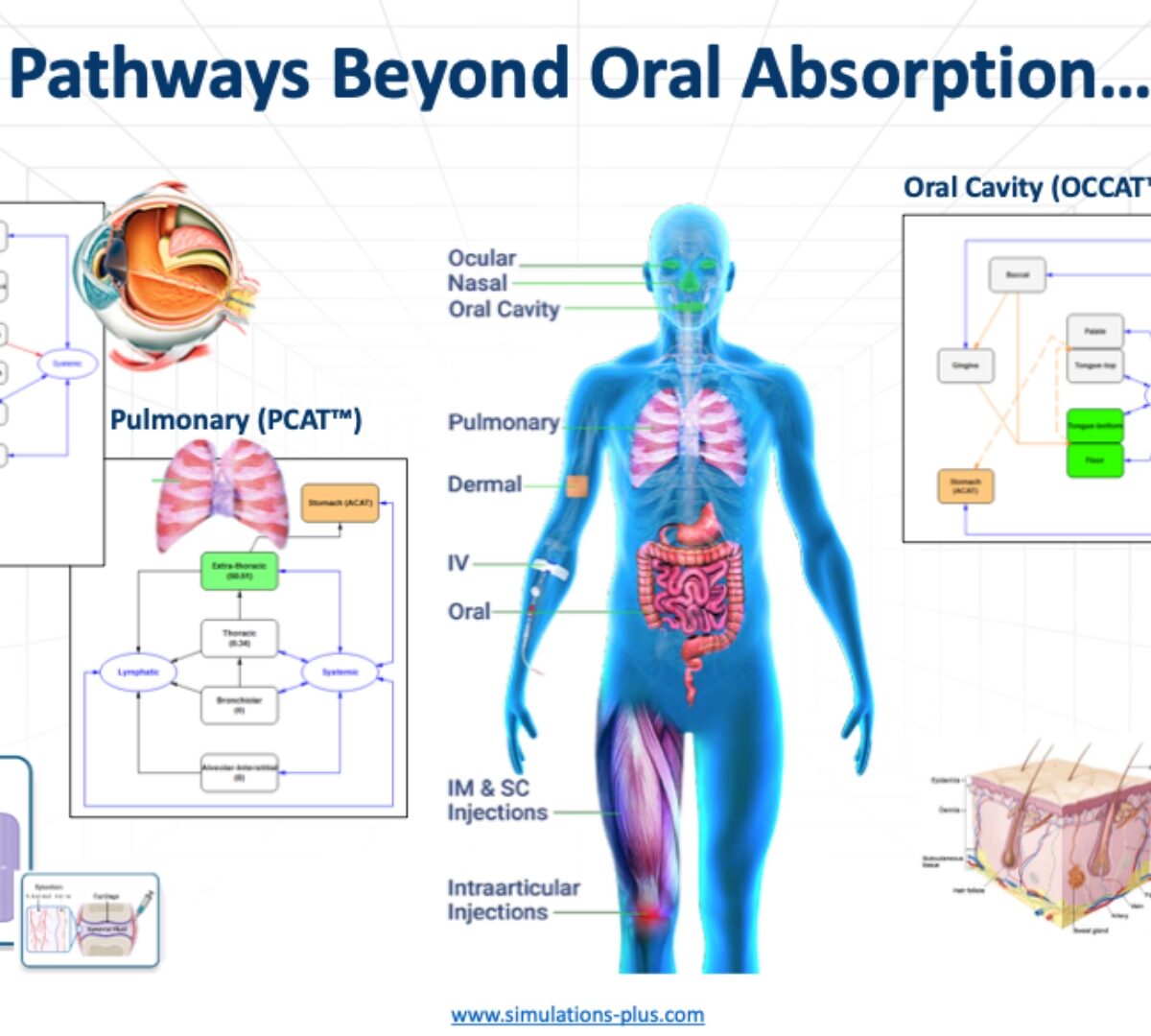
Prediction of tissue and urine concentrations of 2-phenoxyethanol and its metabolite 2-phenoxyacetic acid in rat and human after oral and dermal exposures via GastroPlus™ physiologically based pharmacokinetic modelling
A physiologically based pharmacokinetic (PBPK) model for the important chemical phenoxyethanol (PhE) and its metabolite phenoxyacetic acid (PhAA) was built via GastroPlusTM...

Exploration of the Plausible Mechanism of Ethambutol Induced Ocular Toxicity by Using Proteomics Informed Physiologically Based Pharmacokinetic (PBPK) Modeling
Ethambutol (EMB) is a first-line anti-tubercular drug that is known to cause optic neuropathy.

Use In Silico and In Vitro Methods to Screen Hepatotoxic Chemicals and CYP450 Enzyme Inhibitors
In silico and in vitro methods have emerged as valuable tools to rapidly screen and prioritize large numbers of chemicals including new drug entities, food ingredients, and environmental compounds for further in vivo analysis.
