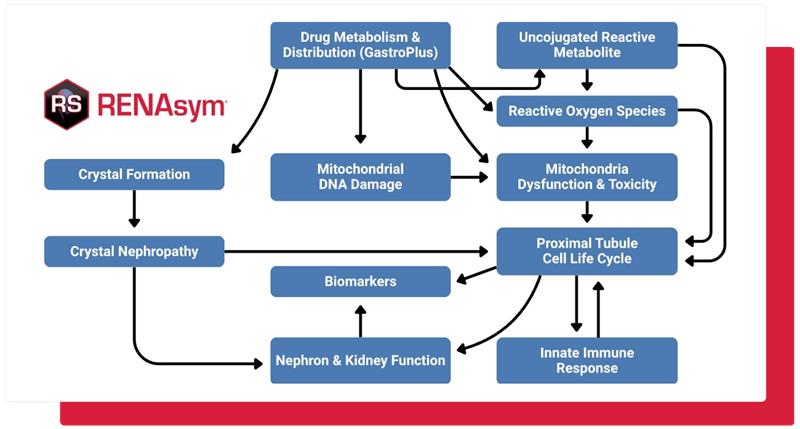
No one wants to risk potential kidney safety issues. RENAsym is a software platform designed to help researchers predict and understand drug-induced kidney injury.
Using mechanistic modeling, RENAsym simulates how drug candidates may impact the kidneys, and predicts the likelihood of renal injury in a patient population under different dosing strategies. It can also provide deeper insight into the biological mechanisms behind observed renal safety signals, leading to more informed drug design and stronger regulatory submissions.