Absorption, distribution and clearance of triamcinolone acetonide (TA) from oral and pulmonary administrations have been simulated using GastroPlus™,1. Simulation of orally administered doses and swallowed…

Mixture Modeling as a Data Imputation Method
To demonstrate the use of mixture modeling in population PK analysis to predict drug concentrations for a subset of subjects with missing data for a key categorical covariate.

A Semi-mechanistic Approach to PK/PD Modeling of Complex Response Data: Bone Turnover Example for Odanacatib, a Cathepsin K Inhibitor
A variety of approaches can be taken to incorporate greater mechanistic understanding into PK/PD models, including a variety of bottom-up, middle-out, and top-down approaches. The osteoporosis field…

New to an Organization: Tools to Assess Projects and Teams
The complexity of project management and its value increase exponentially as the number and complexity of projects and interdisciplinary team participation increases.

Exposure–Response Analysis of Eslicarbazepine Acetate as Adjunctive Treatment of Patients With Partial-onset Seizures
Eslicarbazepine acetate (ESL) is a novel once-daily (QD) antiepileptic drug (AED) currently under clinical development in the US. ESL is rapidly and extensively metabolized to its major active metabolite…

Population Pharmacokinetics of Eslicarbazepine Acetate in Patients With Partial-onset Seizures
Eslicarbazepine acetate (ESL) is a novel once-daily antiepileptic drug (AED) currently under clinical development in the US. Following oral administration, ESL is rapidly and extensively metabolized…

Evolving hERG Inhibition Model
Modeling hERG inhibition has significantly gained popularity since 2005, when the FDA recognized the correlation between hERG inhibition and a prolonged QT interval by issuing guidance for the…

Physiologically-Based Pharmacokinetic (PBPK) Model for Prediction of Tobramycin Pulmonary Absorption and Pharmacokinetics in Children
To fit an absorption-pharmacokinetic model for simulation of tobramycin in adult and pediatric populations. Tobramycin pulmonary absorption and pharmacokinetics were simulated using GastroPlus™.

Physiologically-based pharmacokinetic {PBPK) models for prediction of time-dependent enzyme inhibition (TDI): effect of diltiazem on midazolam and quinidine
Purpose of the study was to optimize a PBPK model to predict timedependent and competitive inhibition of CYP 3A4 by diltiazem...

Development of A Mechanistic in vitro – in vivo Correlation for Theophylline
Theophylline is used to prevent and treat wheezing, shortness of breath, and difficulty breathing caused by asthma, chronic bronchitis, emphesema, and other lung diseases. Immediate release oral…

Application of a Respiratory PBPK Model for Predicting Deposition and Disposition following Inhaled Administration of Morphine
Demonstrate the pulmonary component of the GastroPlus™ Additional Dosage Routes Module™ (ADRM) simulation to develop a pharmacokinetic (PK) and pharmacodynamic (PD) model for inhaled…

Mechanistic PBPK Simulation in Place of In Vivo Drug-Drug Interaction (DDI) Studies for Compliance with Regulatory Requirements of EMA and FDA
To highlight the application and validation of PBPK DDI simulation results obtained using GastroPlus™ in compliance with newly proposed European Medicines Agency (EMA) and US FDA guidelines for the…

Beyond Filters: ADMET Risk™ for Multi-objective Drug Development
We calculated predictions for over 30 properties relevant to absorption, distribution, metabolism, excretion and toxicity (ADMET) for a large and pharmaceutically pertinent...

Forensic Pharmacometrics: Part 2 – Deliverables for Regulatory Submission
As modeling and simulation results become increasingly integral to critical development-related decision-making and program outcomes, the consequences of poor documentation of pharmacometric analyses can…

Physiologically-Based Pharmacokinetic (PBPK) Modeling of Diltiazem-Midazolam Drug-Drug Interaction (DDI)
The purpose of this modeling effort was to explore the effects of various processes and physiological parameters on the DDI involving competitive and time-dependent inhibition (TDI). Absorption and…

Single-Dose And Steady-State Pharmacokinetics Of Moxduo™, A Dual-Opioid Formulation Containing A Fixed Ratio Of Morphine And Oxycodone
Q8003 (MoxDuo™) is the first dual-opioid combination product that has been evaluated in clinical trials. MoxDuo immediate-release capsules were developed for the management of acute moderate to severe…

Semi-mechanistic PK/PD Model of the Effect of Odanacatib, a Cathepsin K Inhibitor, on Bone Turnover to Characterize Lumbar Spine Bone Mineral Density in Two Phase II Studies of Postmenopausal Women
Odanacatib (MK-0822), a potent, orally-active inhibitor of cathepsin K, is under clinical development for treatment of postmenopausal osteoporosis. This poster describes base model development of a…
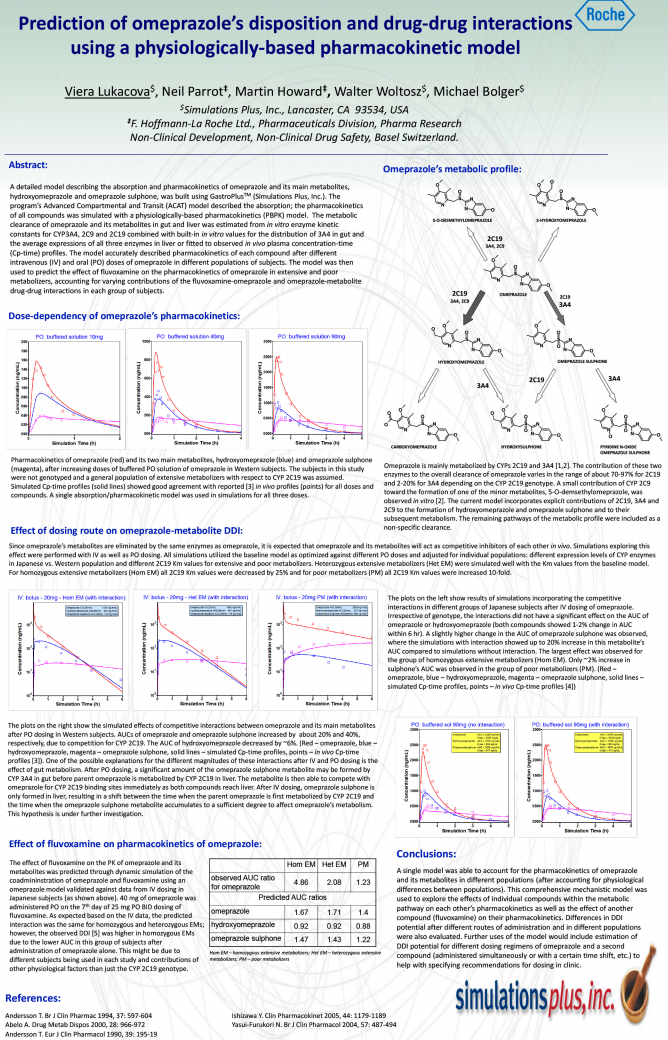
Prediction of Omeprazole’s Disposition and Drug-Drug Interactions Using A Physiologically-Based Pharmacokinetic Model
Download the poster presented at the ADMET Europe 2010 conference on the development of PBPK models and prediction of parent & metabolite DDIs with omeprazole.

Simulations of the Drug-Drug Interaction Between Atomoxetine and Quinidine in Poor and Extensive CYP2D6 Metabolizers
Atomoxetine is indicated for attention-deficit hyperactivity disorder (ADHD) in children, adolescents and adults. It is metabolized to 4- hydroxy-atomoxetine primarily by CYP2D6, which is known to have…

Population Pharmacokinetics of Dexmedetomidine (DEX) During Long-Term Continuous Infusion in Critically Ill Patients
Dexmedetomidine (DEX), a selective alpha2-adrenoceptor agonist is approved for sedation. In this study, the population pharmacokinetics (PK) of DEX during long-term (> 24 hours) infusion was…
