Project provides ~$4.7 million over 5 years to implement a platform for global teams engaged in model-based drug development

Integrating Predictions from Complementary Cytochrome P450 (CYP) Models
Many structure-activity classification models have been published for predicting whether a given compound is likely to inhibit and/or be subject to metabolism by a given cytochrome P450 (CYP) isoform, and...

Febrifugine analogues as Leishmania donovani trypanothione reductase inhibitors: binding energy analysis assisted by molecular docking, ADMET and molecular dynamics simulation
Visceral leishmaniasis affects people from 70 countries worldwide, mostly from Indian, African and south American continent. The increasing resistance to antimonial, miltefosine and frequent toxicity of...

pH-Dependent Solubility and Dissolution Behavior of Carvedilol-Case Example of a Weakly Basic BCS Class II Drug
The objective of this study was to investigate the pH-dependent solubility and dissolution of weakly basic Biopharmaceutical Classification Systems (BCS) class II drugs, characterized by low solubility...

New design of nucleotide excision repair (NER) inihibitors for combination cancer therapy
Many cancer chemotherapy agents act by targeting the DNA of cancer cells, causing substantial damage within their genome and causing them to undergo apoptosis.

Simulated rat intestinal fluid improves oral exposure prediction for poorly soluble compounds over a wide dose range
Solubility can be the absorption limiting factor for drug candidates and is therefore a very important input parameter for oral exposure prediction of compounds with limited solubility.

Advantage of the Dissolution / Permeation System for Estimating Oral Absorption of Drug Candidates in the Drug Discovery Stage
In order to increase the success rate in the development of oral drugs, an in vitro method, which can accurately estimate human oral absorption of a large variety of compounds from solid formulations...
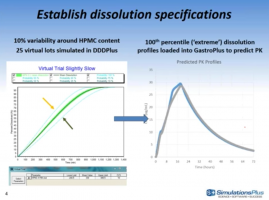
Reimagine the In Vitro Dissolution Experiment with DDDPlus™ 5.0…
In this webinar, case studies will be presented highlighting the use of DDDPlus and GastroPlus to optimize formulations and apply virtual 'lot-to-lot' variability effects to help establish dissolution specifications.

Novel nonquaternary reactivators showing reactivation efficiency for soman-inhibited human acetylcholinesterase
Soman is a highly toxic nerve agent with strong inhibition of acetylcholinesterase (AChE), but of the few reactivators showing antidotal efficiency for soman-inhibited AChE presently are all...

Software and Web Resources for Computer-Aided Molecular Modeling and Drug Discovery
Computer-aided molecular modeling and drug design plays a crucial role in drug discovery and has become an essential tool in the pharmaceutical industry.
![Synthesis and Evaluation of Novel Radioligands Based on 3-[5-(Pyridin-2-yl)-2H-tetrazol-2-yl]benzonitrile for Positron Emission Tomography Imaging of Metabotropic Glutamate Receptor Subtype 5](https://www.simulations-plus.com/wp-content/themes/simulations-plus/library/dist/img/default_square-large.jpg)
Synthesis and Evaluation of Novel Radioligands Based on 3-[5-(Pyridin-2-yl)-2H-tetrazol-2-yl]benzonitrile for Positron Emission Tomography Imaging of Metabotropic Glutamate Receptor Subtype 5
We found out 3-[5-(pyridin-2-yl)-2H-tetrazol-2-yl]benzonitrile analogues as the candidate for positron emission tomography (PET) imaging agents of metabotropic glutamate receptor subtype 5 (mGluR5).

Introduction to Cheminformatics
Cheminformatics is a field of information technology that focuses on the collection, storage, analysis, and manipulation of chemical data.

Development of 3 , 5-Dinitrobenzylsulfanyl-1, 3, 4-Oxadiazoles and Thiadiazoles as Selective Antitubercular Agents Active Against Replicating and Nonreplicating Mycobacterium tuberculosis
Herein, we report the discovery and structure–activity relationships of 5-substituted-2-[(3,5-dinitrobenzyl)sulfanyl]-1,3,4-oxadiazoles and 1,3,4-thiadiazoles as a new class of antituberculosis agents.

A review of the current state of the art of physiologically-based tests for measuring human dermal in vitro bioavailability of polycyclic aromatic hydrocarbons (PAH) in soil
Polycyclic Aromatic Hydrocarbons are classed as Persistent Organic Pollutants, a large group of compounds that share similar characteristics.

Simulations Plus Releases KIWI Version 1.5
A new modeling visualization experience

QSAR-based Prediction of Ames Mutagenicity for ICH M7 Submissions
The “Ames test”, originally developed by Bruce Ames and his group, is a way to measure the mutagenic potential of chemicals.1 It uses strains of Salmonella typhimurium and Escherichia coli as an...
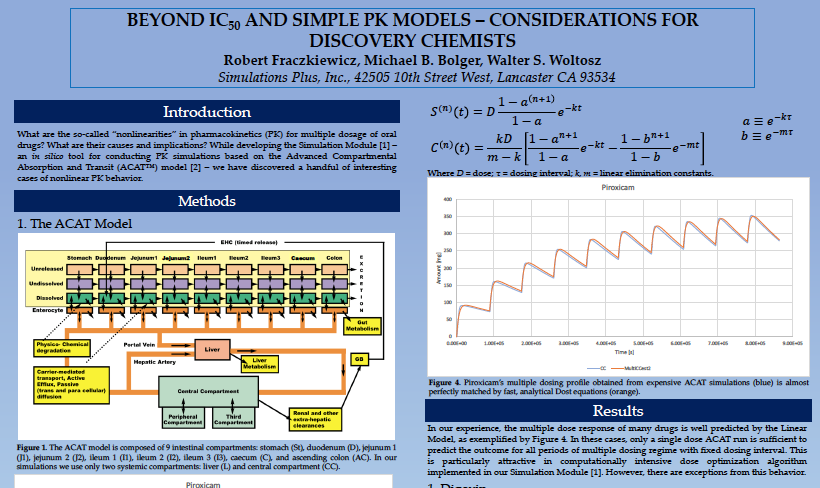
Beyond IC50 and simple PK models – Considerations for discovery chemists
While developing the Simulation Module [1] – an in silico tool for conducting PK simulations based on the Advanced Compartmental Absorption and Transit (ACAT™) model [2] – we have discovered a handful of...

Innate immune signaling through differential RIPK1 expression promote tumor progression in head and neck squamous cell carcinoma
Head and neck squamous cell carcinoma (HNSCC) is a devastating disease for which new treatments, such as immunotherapy are needed.

Mechanistic Modeling Predicts Drug-Induced HyperbilirubinemiaThat Involves Inhibition Of Enzymes And Transporters
Elevated serum ALT and bilirubin indicates high risk of fatal drug-induced liver injury. However, drugs also can increase serum bilirubin in the absence of hepatic injury by inhibiting enzymes and/or transporters.
