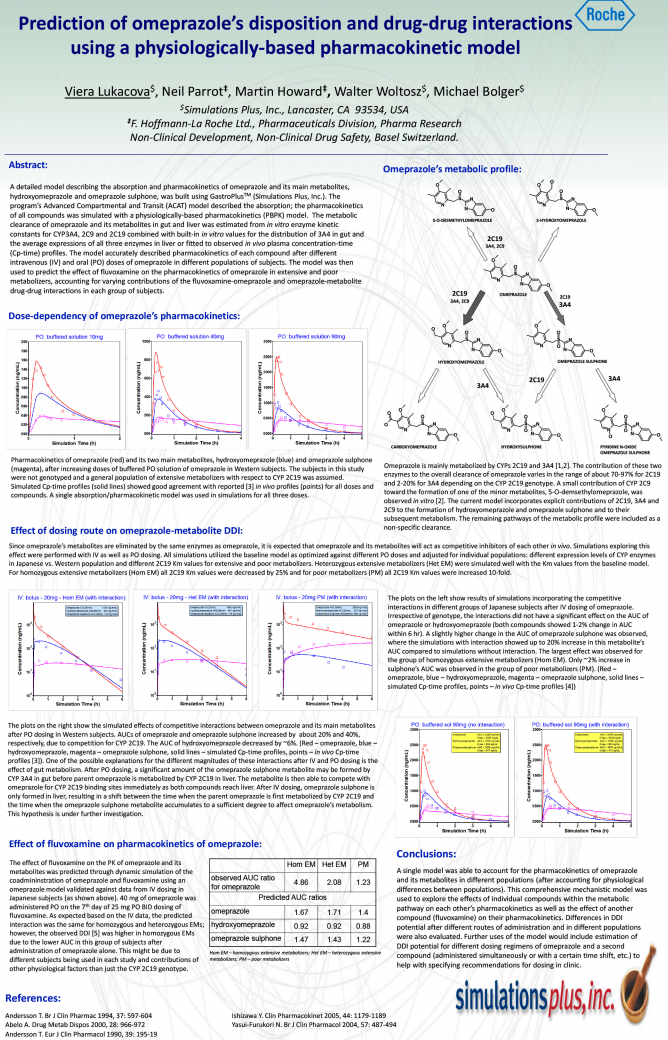
Download the poster presented at the ADMET Europe 2010 conference on the development of PBPK models and prediction of parent & metabolite DDIs with omeprazole.

Simulations of the Drug-Drug Interaction Between Atomoxetine and Quinidine in Poor and Extensive CYP2D6 Metabolizers
Atomoxetine is indicated for attention-deficit hyperactivity disorder (ADHD) in children, adolescents and adults. It is metabolized to 4- hydroxy-atomoxetine primarily by CYP2D6, which is known to have…

AR inhibitors identified by high-throughput microscopy detection of conformational change and subcellular localization
Signaling via the androgen receptor (AR) plays an important role in human health and disease. All currently available anti-androgens prevent ligand access to the receptor...

Population Pharmacokinetics of Dexmedetomidine (DEX) During Long-Term Continuous Infusion in Critically Ill Patients
Dexmedetomidine (DEX), a selective alpha2-adrenoceptor agonist is approved for sedation. In this study, the population pharmacokinetics (PK) of DEX during long-term (> 24 hours) infusion was…

Quantitative Prediction of Regioselectivity Toward Cytochrome P450/3A4 Using Machine Learning Approaches
In the drug discovery process, it is important to know the properties of both drug candidates and their metabolites.

A Systems Approach to Resource Allocation in an Integrated Research and Development Environment
Typically, at the beginning of a project, the scope is defined along with the timelines and budget. As project team leaders negotiate with functional managers to recruit key team members, including…

Lean Production
I have been reading The Machine That Changed the World: The Story of Lean Production (1), by James Womack and others from MIT’s International Motor Vehicle Program (Content no longer available) research team. This book caused a sensation 20 years ago with its description of the Toyota Production System. The blurb on the book’s back cover says, “The hallmarks of lean production are teamwork, communication, and efficient use of resources. The results are remarkable cars with one-third the defects, built in half the factory space, using half the man-hours.”

Semi-mechanistic PK/PD Model of the Effect of Odanacatib, a Cathepsin K Inhibitor, on Bone Turnover to Characterize Lumbar Spine Bone Mineral Density in Two Phase II Studies of Postmenopausal Women
Odanacatib (MK-0822), a potent, orally-active inhibitor of cathepsin K, is under clinical development for treatment of postmenopausal osteoporosis. This poster describes base model development of a...

Simulations Plus Launches Biorelevant Solubility Database
Proprietary Data Generated by Company Provides Unique Insight Needed By Drug Developers

Simulations Plus Announces Renewed Share Repurchase Program
Up to One Million Shares over 12 Months Authorized by Board of Directors

Intro to Pharma of the Future?
Why is it so hard to incorporate modeling and simulation into drug development? Why do these powerful tools so often fail to provide satisfactory outcomes?

Simulations Plus Reports First Quarter FY2010 Financial Results
Record First Quarter Sees Pharmaceutical Software and Services Revenues Jump 21%, Consolidated Earnings Up 38% from 1QFY09

Paul Volcker: Think More Boldly
In December 2009, The Wall Street Journal sponsored its second Future of Finance Initiative (links to a dead page) to provide a forum for 80 of the world’s top financiers to brainstorm suggestions for reforming the financial system in the wake of the 2008 implosion of the global economy.

Development of a Physiologically Based Pharmacokinetic (PBPK) Model for Predicting Deposition and Disposition follo · g Inhaled and Intranasal Administration
The selection of a biologically active molecule as a successful inhaled therapeutic agent depends on its pharmacokinetic and safety properties...

Simulations Plus Appoints John DiBella, M.S., Manager, Marketing and Sales
New Leadership Brings Strength in Science and Engineering

Simulations Plus Appoints Robert D. Clark, PH. D., Director of Life Sciences
Dr. Clark to Join Team in January

Simulations Plus Launches Abbreviate!™ for the iPhone™
New Software Speeds Up Typing on the PopularApple® iPhoneTM and iPod Touch®