Dissolution limitations to oral absorption can occur if the time required for dissolution is longer than the transit time across the small intestine and/or if dissolution is slower than the drug’s permeation through the gut wall.

Physiologically based pharmacokinetic (PBPK) modelling of oral drug absorption in older adults – an AGePOP review
The older population consisting of persons aged 65 years or older is the fastest-growing population group and also the major consumer of pharmaceutical products.

In silico modeling as a support tool in the development of new products in the generic pharmaceutical industry: case studies of the application of DDDPlus™ and GastroPlus® software
During this webinar, we will shed light on the key benefits and practical implications of utilizing in silico modeling for formulation optimization, biopharmaceutical predictions, and bioequivalence studies

Biopolymer-Capped Pyrazinamide-Loaded Colloidosomes: In Vitro Characterization and Bioavailability Studies
This study aimed to prepare colloidosome particles loaded with pyrazinamide (PZA).

Simulations Plus Reports Third Quarter Fiscal 2023 Financial Results
Total revenue of $16.2 million and diluted earnings per share (EPS) of $0.20
Maintains full-year revenue guidance of $59.3 - $62.0 million (+10-15%) and EPS guidance of $0.63 - $0.67

PBPK Modeling as an Alternative Method of Interspecies Extrapolation that Reduces the Use of Animals: A Systematic Review
Physiologically based pharmacokinetic (PBPK) modeling is a computational approach that simulates the anatomical structure of the studied species...

July 2023 News/Events
Expanding our Immunology and Oncology Drug Development Capabilities

Integrated in vitro – in vivo – in silico studies in the pharmaceutical development of propranolol hydrochloride mucoadhesive buccal films
In order to exploit the advantage of drug delivery through the buccal mucosa, mucoadhesive buccal films with propranolol hydrochloride based on polyethylene oxide...

Consult & Coach Comparison Flyer
Accelerate your project goals with a free 3-month license to Monolix and/or GastroPlus
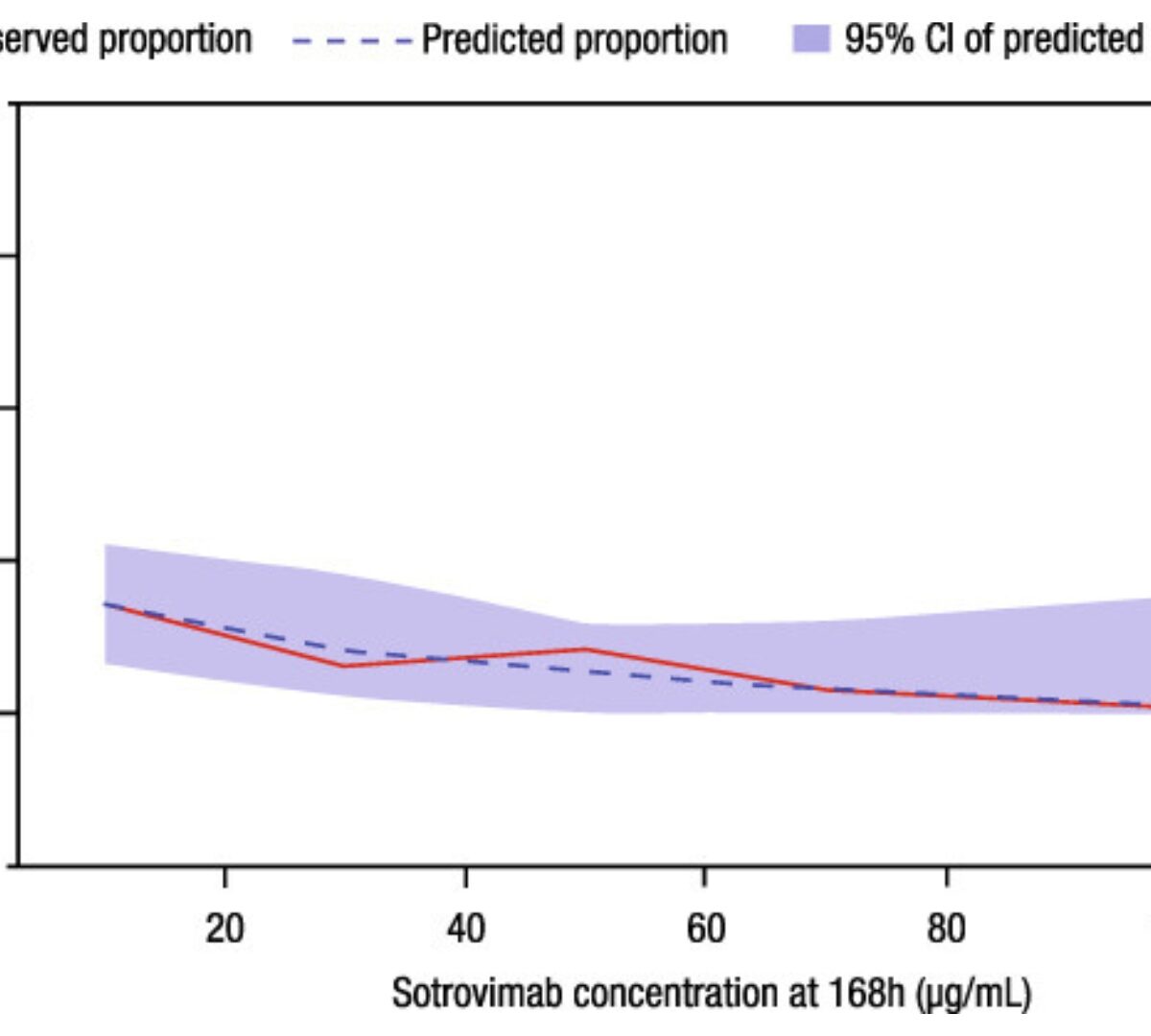
Population Pharmacokinetics and Exposure-Response Analysis of a Single Dose of Sotrovimab in the Early Treatment of Patients With Mild to Moderate COVID-19
Sotrovimab is a recombinant human monoclonal antibody that has been shown to prevent progression to hospitalization or death in non-hospitalized high-risk patients with mild to moderate...
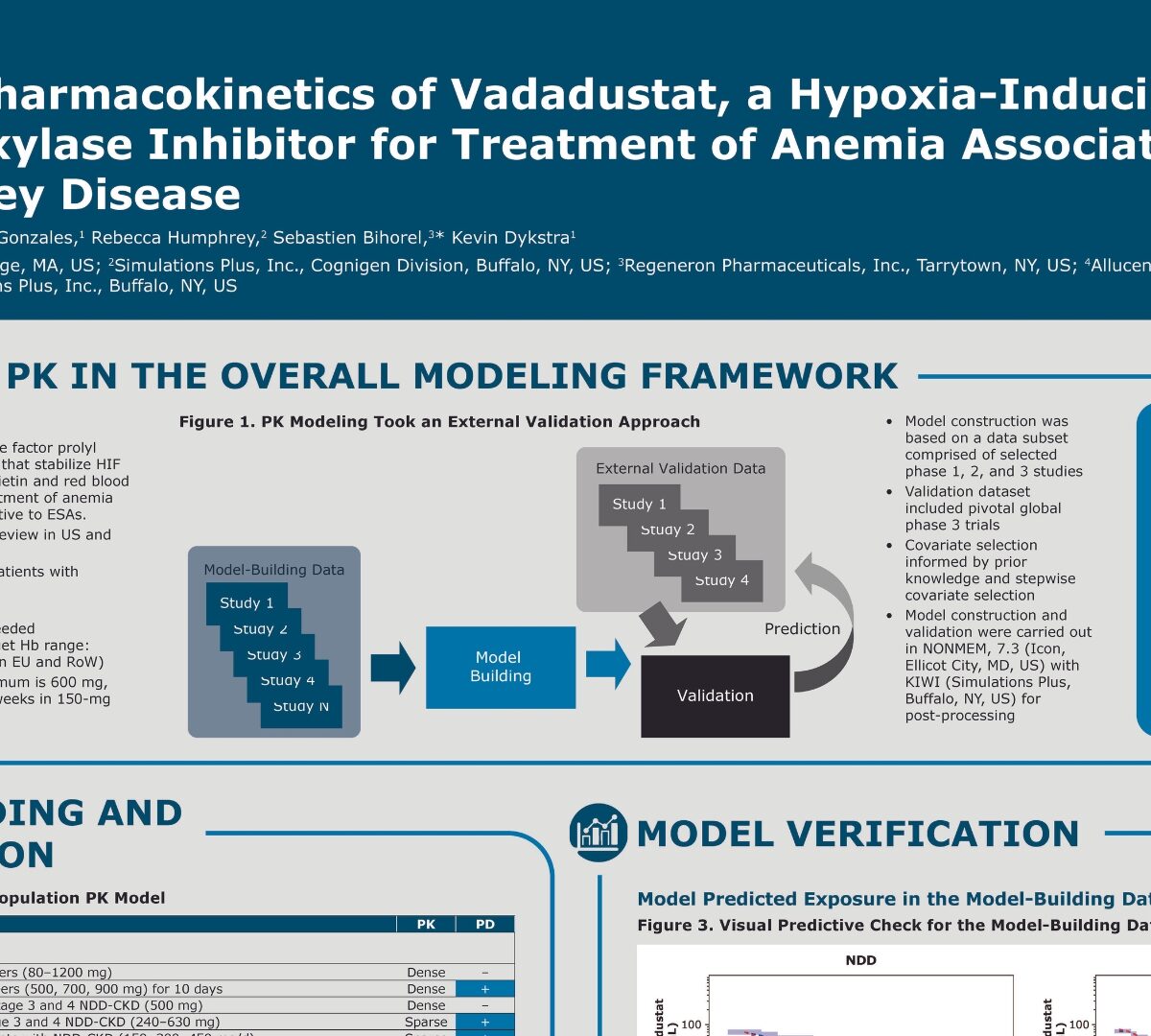
Population pharmacokinetics of vadadustat, a hypoxia-inducible factor prolyl hydroxylase inhibitor for treatment of anemia associated with chronic kidney disease
Vadadustat is an oral hypoxia-inducible factor prolyl hydroxylase inhibitor, a class of...
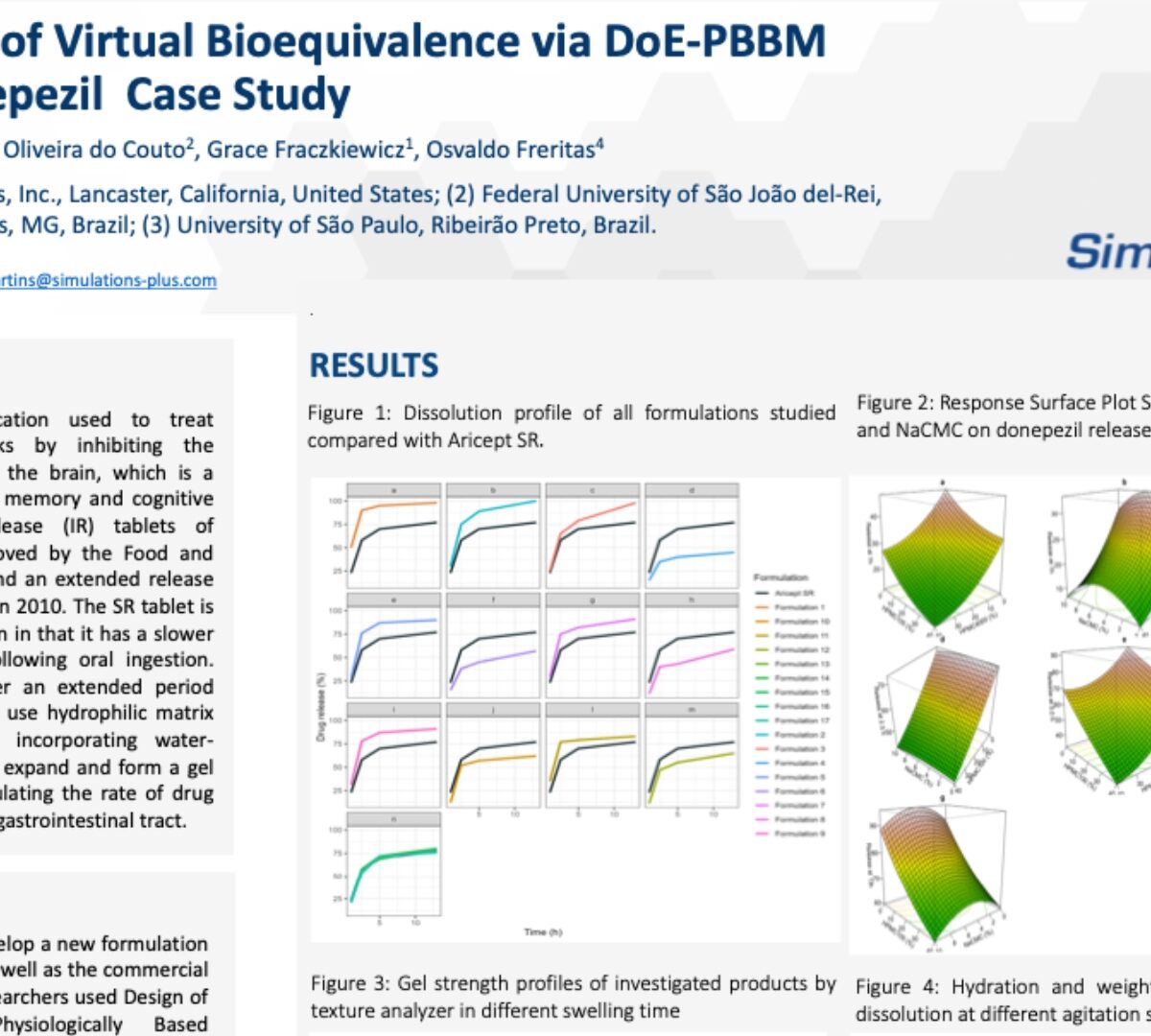
Establishment of Virtual Bioequivalence via DoE-PBBM Model: A Donepezil Case Study
Donepezil (DZP) is a medication used to treat Alzheimer's disease.

ADMET Predictor® 11 is Here: AI & ML for Drug Discovery and Development
Your competitors are using AI and machine learning for ADMET prediction—are you?

AI-Driven Drug Design (AIDD) Flyer
Drug Design, Meet PBPK

Early Drug Discovery (EDD) Flyer
Unleash the Power of AI for Early Drug Discovery

Revisiting the in-vitro and in-vivo considerations for in-silico modelling of complex injectable drug products
Complex injectable drug products (CIDPs) have often been developed to modulate the pharmacokinetics along with efficacy for therapeutic agents used for remediation of chronic disorders.

Modeling Based Approaches to Support Generic Drug Regulatory Submissions-Practical Considerations and Case Studies
Model informed drug development (MiDD) is useful to predict in vivo exposure of drugs during various stages of the drug development process. This approach employs a variety of quantitative tools to assess the risks during the drug development process.

Simulations Plus Acquires Immunetrics to Expand its Immunology and Oncology Drug Development Capabilities
Acquisition increases breadth and depth of QSP expertise and range of therapeutic applications