
Development of an Improved Dissolution Testing Method as an Alternative to Animal Testing
This study aimed to evaluate the potential of an improved flow-through cell dissolution method as an alternative to animal testing for predicting the in vivo behavior of immediate-release formulations.

Integrating PBPK Modeling and Developability Assessment to Guide Early Phase Formulation Strategy
Physiologically based pharmacokinetic (PBPK) modeling is a core element of the early drug and formulation development workflow, enabling data-driven decisions that accelerate timelines and de-risk programs.

Derisking Formulation Strategies Using PBBM: Mechanistic Case Studies on Evaluating Food Effects and Guiding Dissolution Specifications
Understand how PBBM mechanistically integrates formulation dependent processes—such as dissolution, precipitation, gastrointestinal transit, and metabolism—to simulate oral drug absorption under varying prandial conditions.

GastroPlus® X.2: The Deep Dive Webinar Series – How New AI-Powered Tools Can Support Your PBPK Modeling
What if you could design a complex workflow, then set it into motion with a single click?

Use of PBBM-PBPK To Predict Mesalamine Delayed-Release Oral Drug Products Performance in Both Healthy and Disease Physiologies
Mesalamine (5-aminosalicylic acid (5-ASA)) is an anti-inflammatory drug indicated for the treatment of ulcerative colitis (UC) and Crohn’s disease (CD).

From In Vitro Dissolution Testing to In Vivo Clinical Pharmacokinetic Prediction Using PBPK Models for Oral Cavity Drug Products
This work develops a novel in vitro to in vivo extrapolation (IVIVE) method for the prediction of in vivo pharmacokinetic (PK) for oral cavity drug products (DP).

PBPK-Based Prediction of Obesity-Mediated Changes in Small Molecule Pharmacokinetics
Obesity is associated with fat accumulation, combined with physiological alterations, (changes in organ sizes, blood flows, and metabolic enzyme activities).

Predicting Aflibercept Local Exposure in Humans Using an Ocular PBPK Model
Developing both innovative and generic ophthalmic drug products remains a significant hurdle for the pharmaceutical industry.

From Bench to Computational Modeling: Integrating Mitochondrial Stress Data from a Rat Hepatic Ischemia-Reperfusion Injury Model into a Preclinical and Human Quantitative Systems Toxicology Platform
Hepatic ischemia-reperfusion injury (IRI) contributes significantly to liver dysfunction post-surgery and transplantation

Enhancing Drug Formulation Development Through PBBM and GastroPlus®
As anyone who has worked in the pharmaceutical industry knows, there is a constant push to accelerate and optimize development processes.
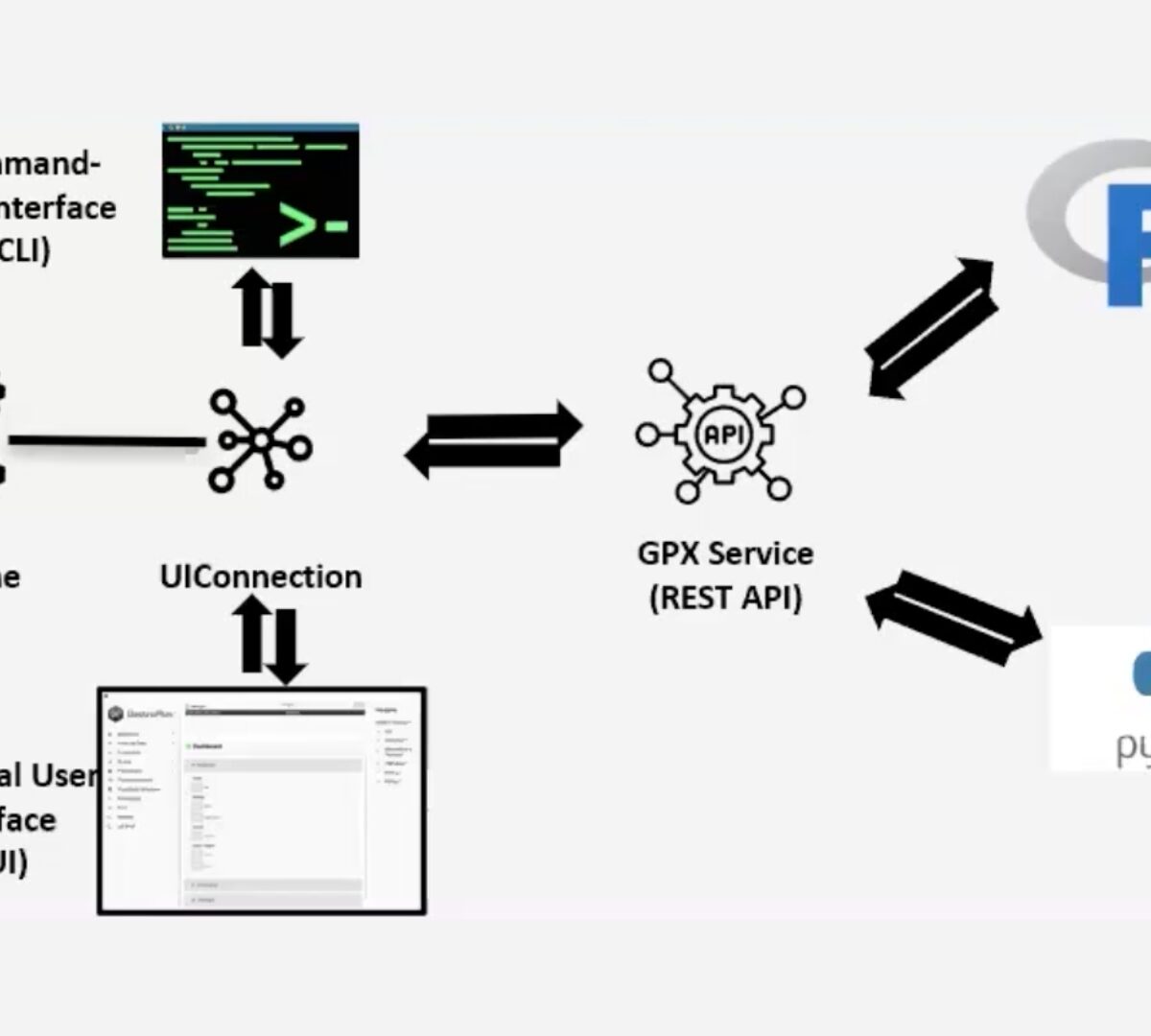
GastroPlus® X.2: The Deep Dive Webinar Series – Introducing Orchestrator, Automation for Complex PBPK/PBBM Modeling in GPX.2
What if you could design a complex workflow, then set it into motion with a single click?

Exploring Artificial Intelligence’s Potential to Enhance Conventional Anticancer Drug Development
Cancer affects one in three to four people globally, with over 20 million new cases and 10 million deaths annually, projected to rise to 35 million cases by 2050. Developing effective cancer treatments is crucial, but the drug discovery process is a highly complex and expensive endeavor, with success rates sitting well below 10% for oncologic therapies

Mechanistic Pharmacokinetic Models for Inhaled Drug Development: Pulmonary Physiological and Structural Characteristics and Modeling Approaches
Pulmonary drug delivery via inhalation offers significant clinical benefits including rapid onset of action and targeted drug administration, which minimizes systemic adverse effects.

Simulated Pharmacokinetic Compatibility of Tamoxifen and Estradiol: Insights from a PBPK Model in Hormone-Responsive Breast Cancer
Although traditionally contraindicated, the coadministration of tamoxifen and estradiol may hold clinical relevance in specific contexts, particularly in breast cancer survivors with premature menopause...
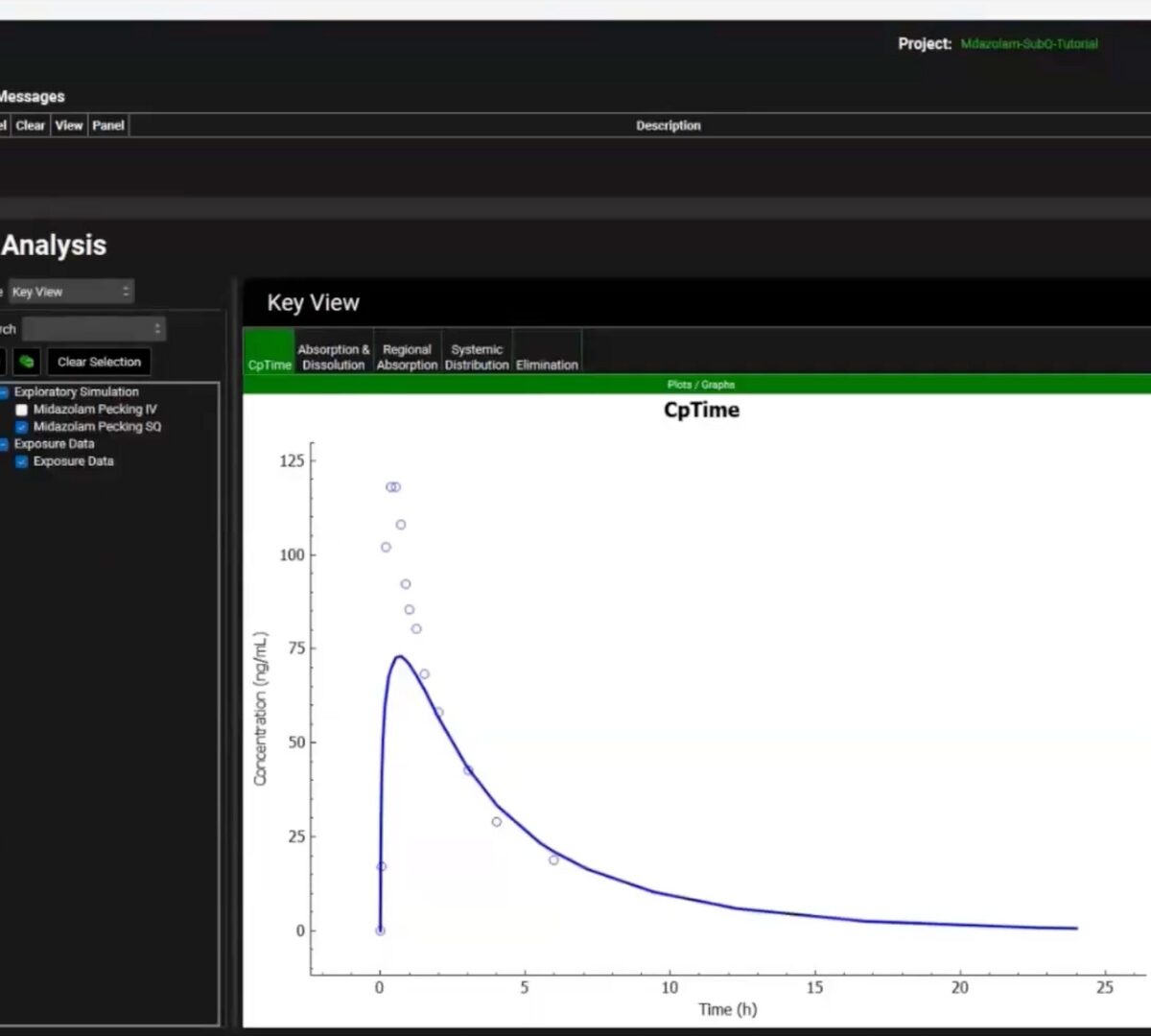
Get Under the Skin with GPX.2
If you’re working on the development of injectable therapies, then this webinar is for you

Introducing Orchestrator – Faster Workflows, Smarter Science
As scientists working in pre-clinical drug development, we are constantly balancing the need for rigorous, mechanistic modeling with the realities of fast-moving project timelines.
![Whole-Body Disposition and Metabolism of [14C]-2,4,4′-Trichlorobiphenyl (PCB28) Following Lung Administration in Rats](https://www.simulations-plus.com/wp-content/themes/simulations-plus/library/dist/img/default_square-large.jpg)
Whole-Body Disposition and Metabolism of [14C]-2,4,4′-Trichlorobiphenyl (PCB28) Following Lung Administration in Rats
Toxicities of lower-chlorinated biphenyls (LC-PCBs) have drawn increasing attention due to growing evidence of their presence in school indoor air, with 2,4,4′-trichlorobiphenyl (PCB28) being a prevalent congener.

Novel Workflow for Non-Animal PBK Modelling of UV Filters: Oxybenzone as a Case Study
Physiologically based kinetics (PBK) modelling provides (internal) exposure concentrations. We used a PBK model parameterized exclusively with in silico and in vitro data in a bottom-up approach to predict the pharmacokinetics of oxybenzone, a UV filter, present in two formulations (for which dose-normalized Cmax and AUC from clinical studies were different).

Simulations Plus Announces Preliminary Fiscal Year 2025 Results and Fiscal Year 2026 Guidance
Provides preliminary fiscal 2026 revenue guidance of $79 to $82 million and adjusted diluted EPS guidance of $1.03 to $1.10
Fourth quarter and fiscal year 2025 results to be reported December 1, 2025
