Q&A presented at SLP MIDD+ Virtual Conference February 16th-17th, 2022
Post Approval: Generics Track – Assessing the Potential for Hepatotoxicity for Combination Therapy of Valproate (VPA) and CBD using Quantitative Systems Toxicology (QST)
Presented by Vinal Lakhani at SLP MIDD+ Virtual Conference February 16th-17th, 2022
Clinical Track: Pushing the Boundaries Beyond – NextGen Session Panel Discussion and Live Q&A
Q&A presented at SLP MIDD+ Virtual Conference February 16th-17th, 2022
Clinical Track Poster Presentation: Count data model for Alzheimer disease progression MMSE score using MonolixSuite®
Presented by Pauline Traynard at SLP MIDD+ Virtual Conference February 16th-17th, 2022
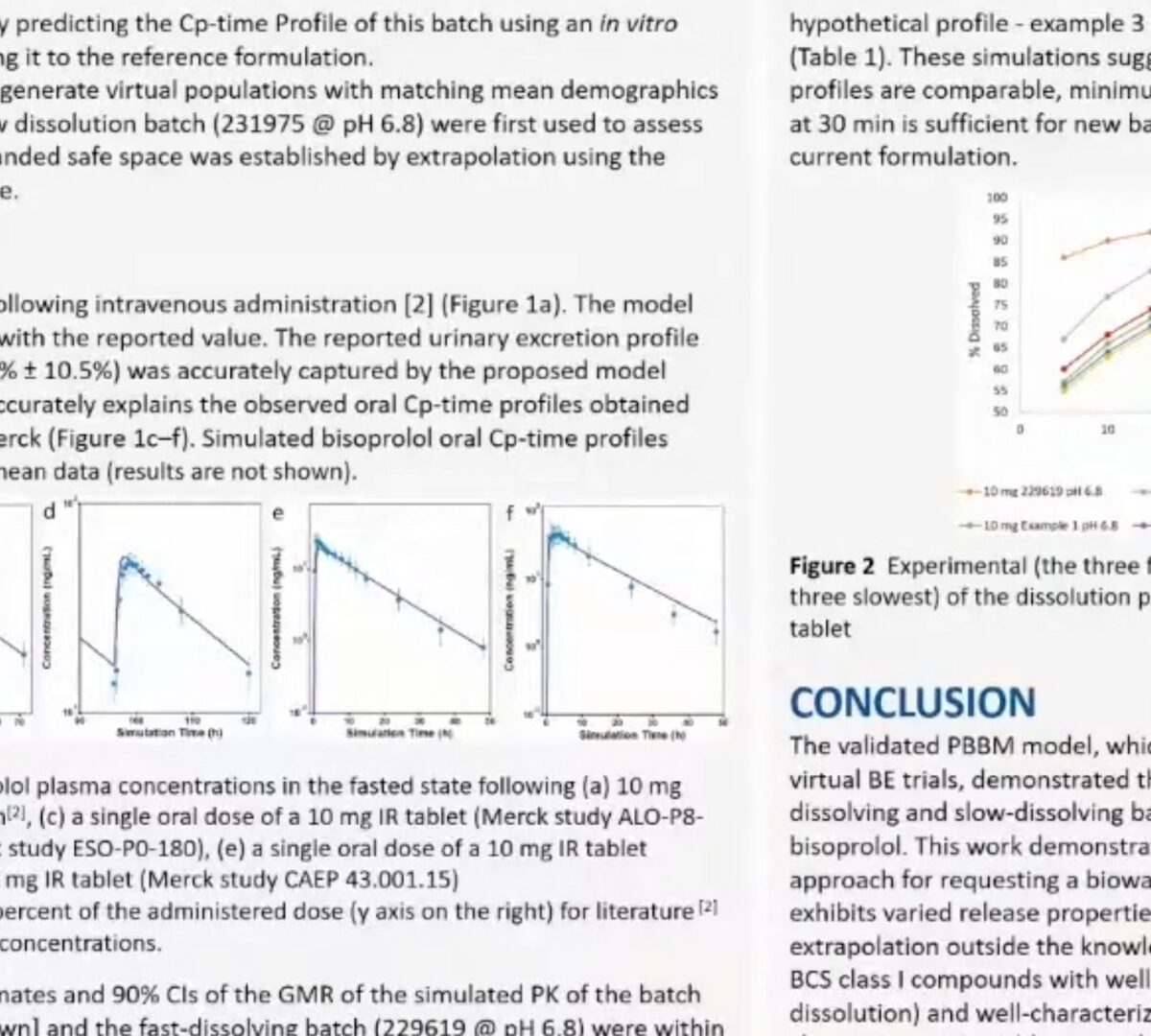
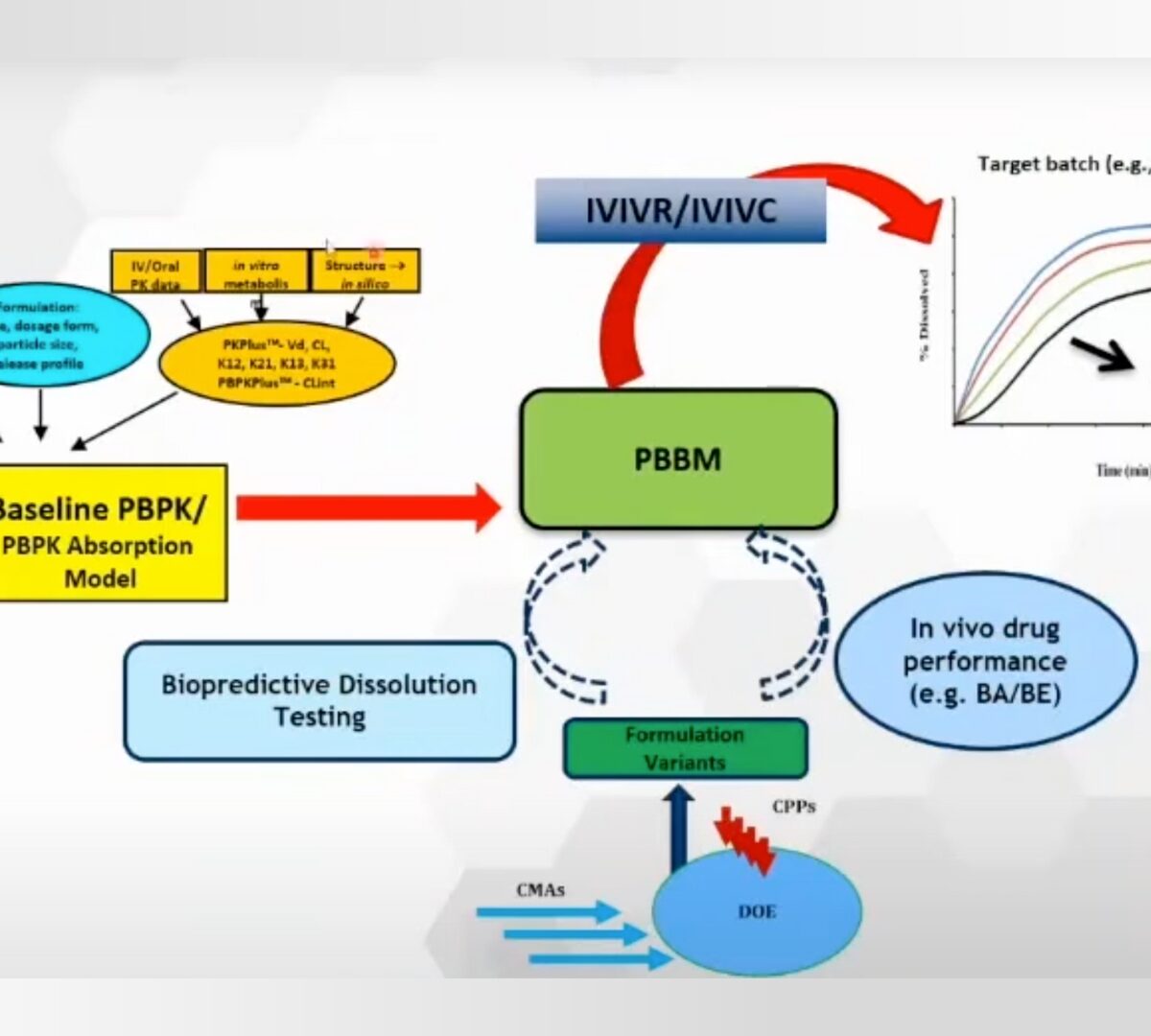
Post-Approval/Generics Track Poster Presentation: Use of PBBM to Establish Dissolution Safe Space via IVIVR for Concor®
Presented by Joyce Macwan at SLP MIDD+ Virtual Conference February 16th-17th, 2022
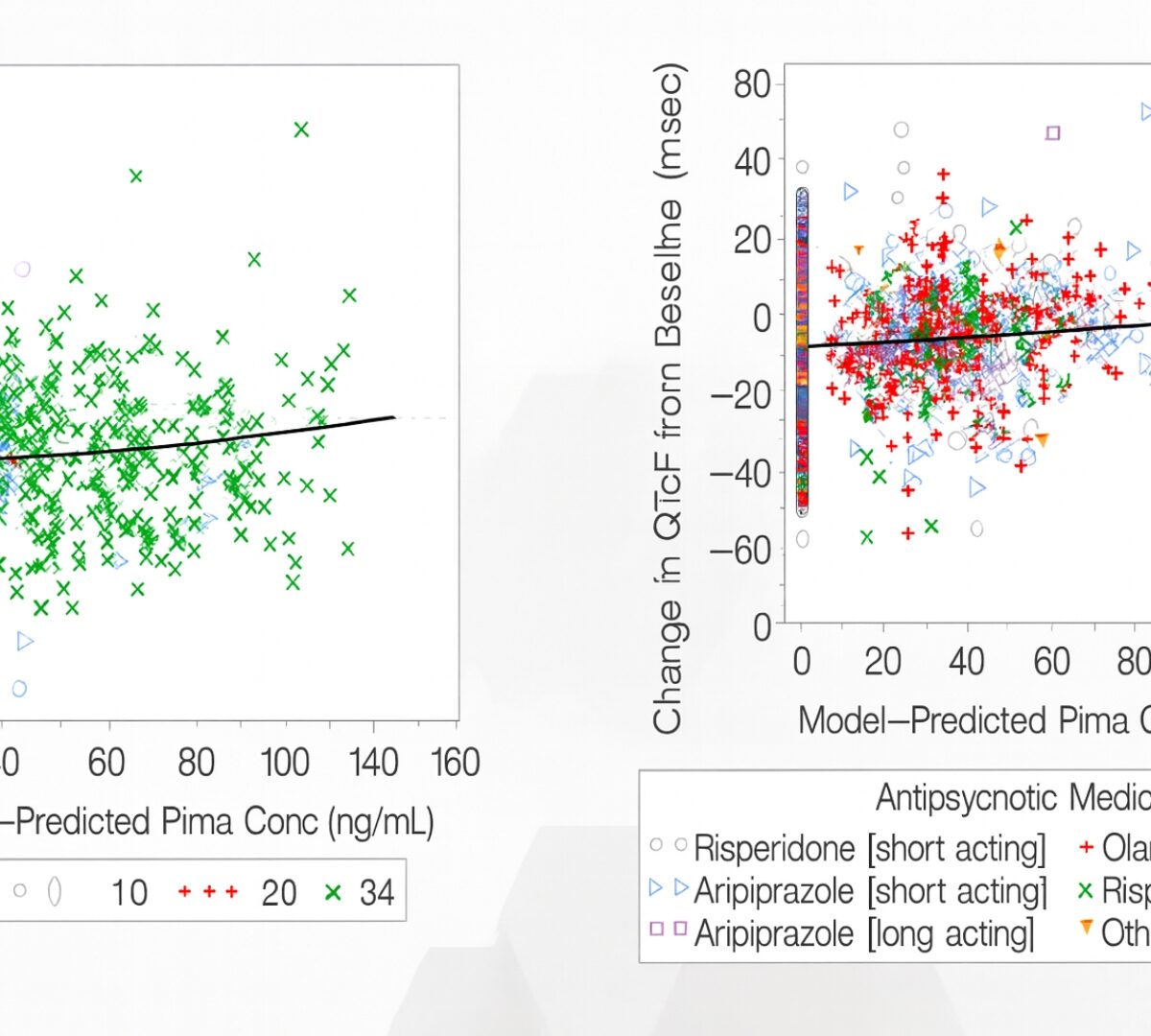
Post-Approval/Generics Track Poster Presentation: Relationship between Pimavanserin Exposure and QTc Interval in Patients with Schizophrenia: Modelling Analysis from Randomized, Double-Blind, Placebo-Controlled Studies
Presented by Hannah Huang at SLP MIDD+ Virtual Conference February 16th-17th, 2022
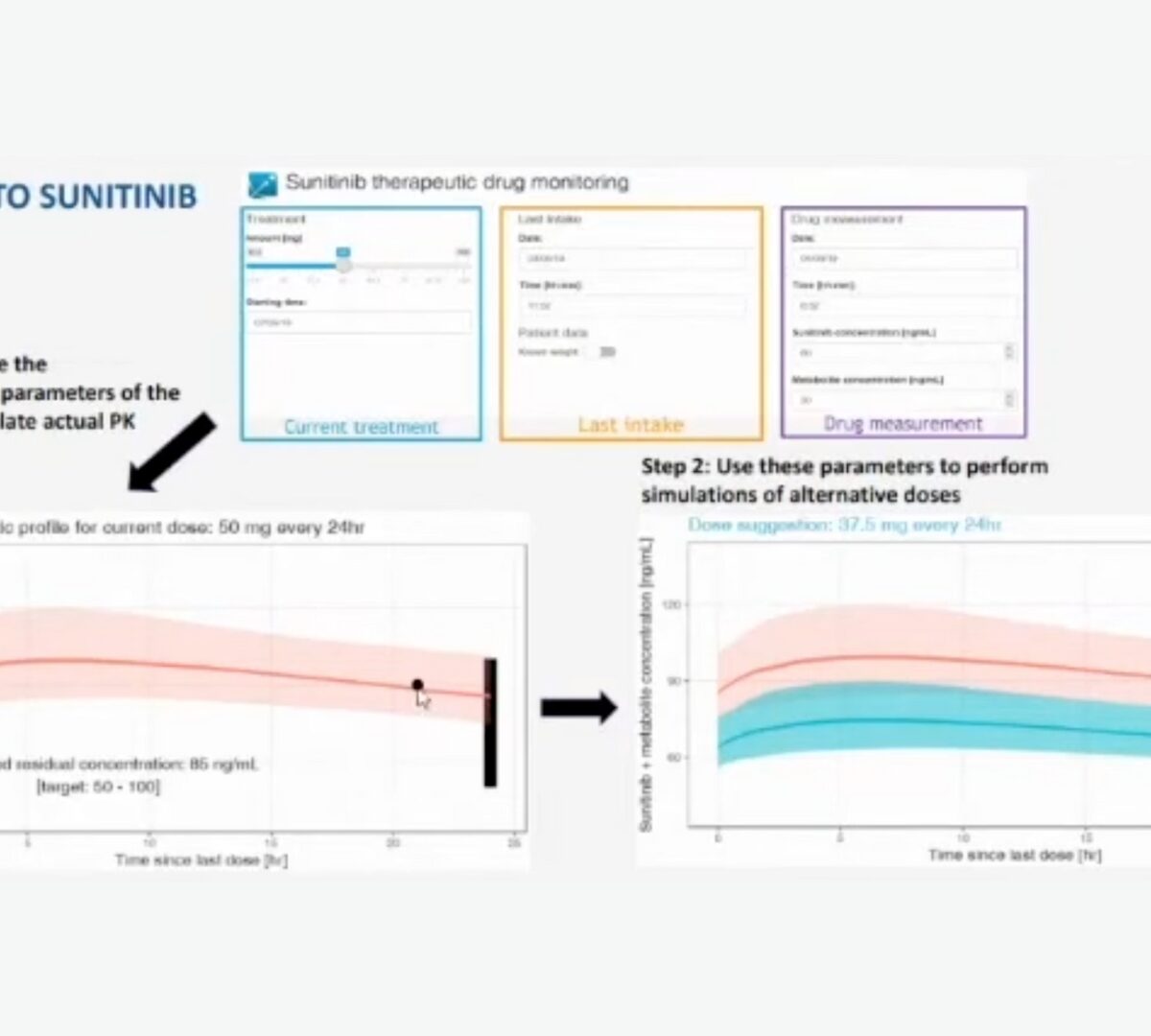
Post-Approval/Generics Track Poster Presentation: Therapeutic Drug Monitoring for Tyrosine Kinase Inhibitors. Possibilities, difficulties, and challenges
Presented by Jonathan Chauvin at SLP MIDD+ Virtual Conference February 16th-17th, 2022

Clinical Track Poster Presentation: Clinical Ocular Exposure Extrapolation Using PBPK Modeling & Simulation: Levofloxacin Solution Case Study
Presented by Maxime LeMerdy at SLP MIDD+ Virtual Conference February 16th-17th, 2022
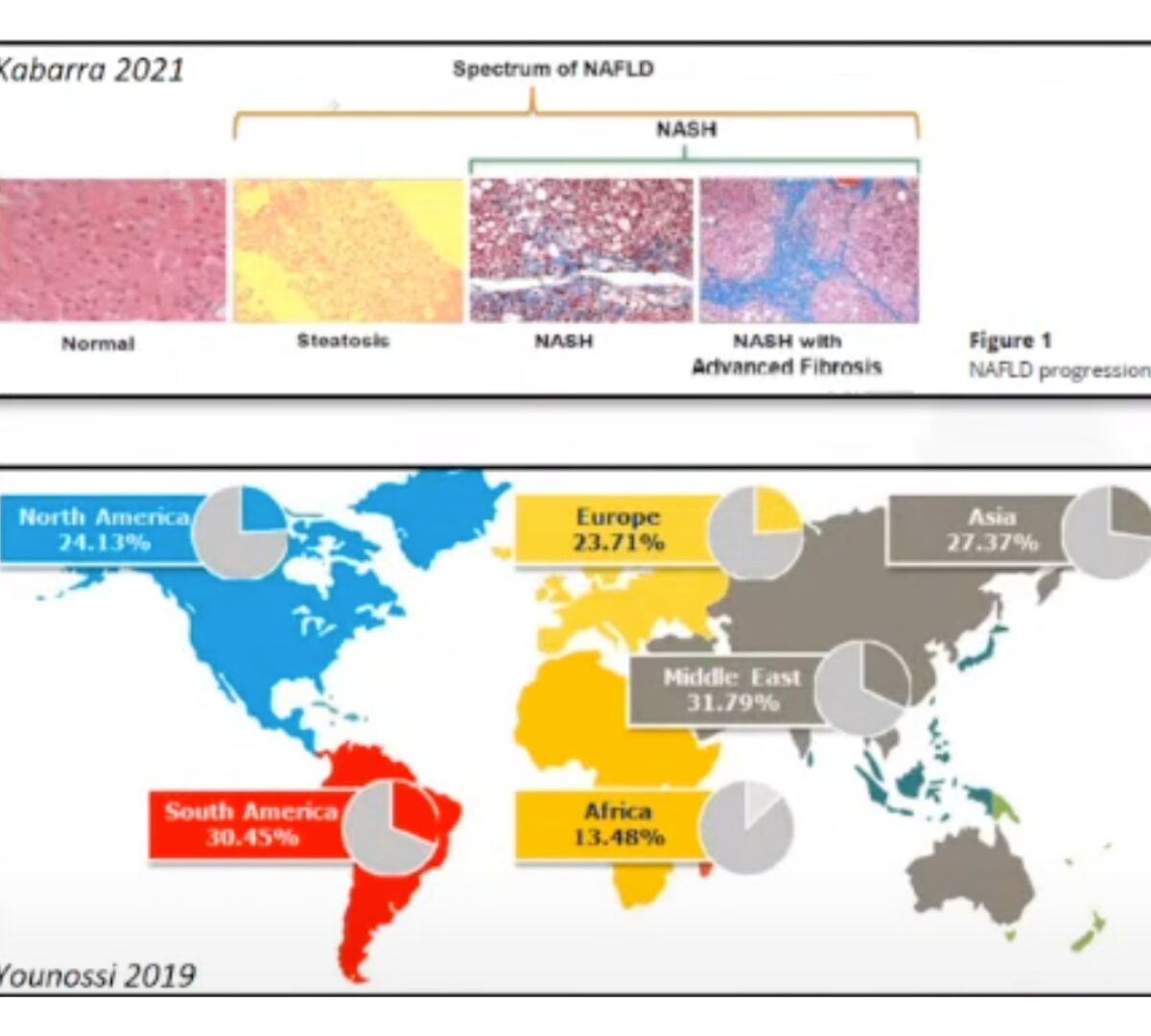
Clinical Track: Yo-Yo Dieting Predicted to Contribute to Fibrosis Score Reductions in Untreated (Placebo) Cohorts
Presented by Lisl Shoda at SLP MIDD+ Virtual Conference February 16th-17th, 2022
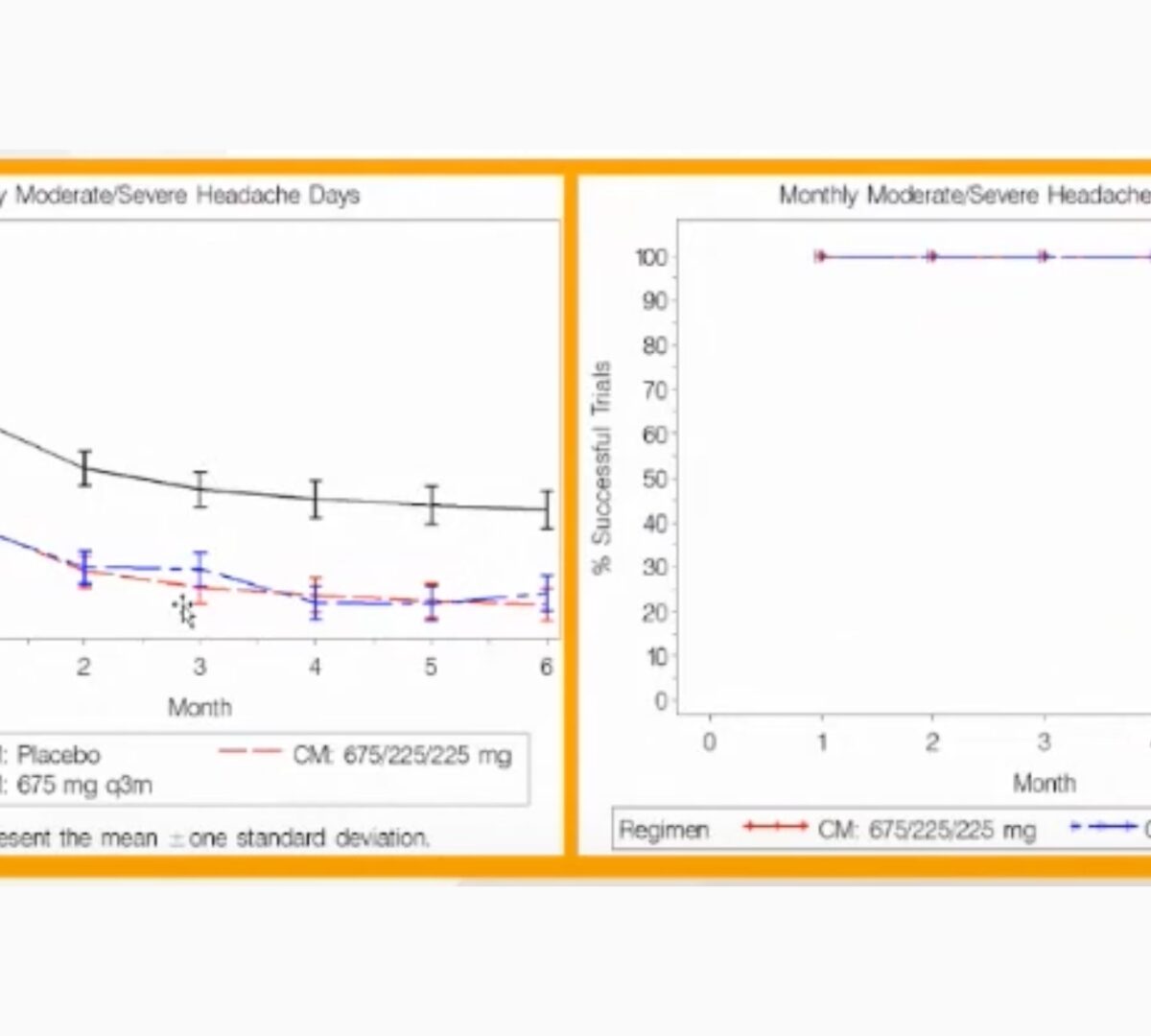
Post-Approval/Generics Track: Use of Exposure-Response Modeling to Support Regulatory Submission
Presented by Julie Passarell at SLP MIDD+ Virtual Conference February 16th-17th, 2022
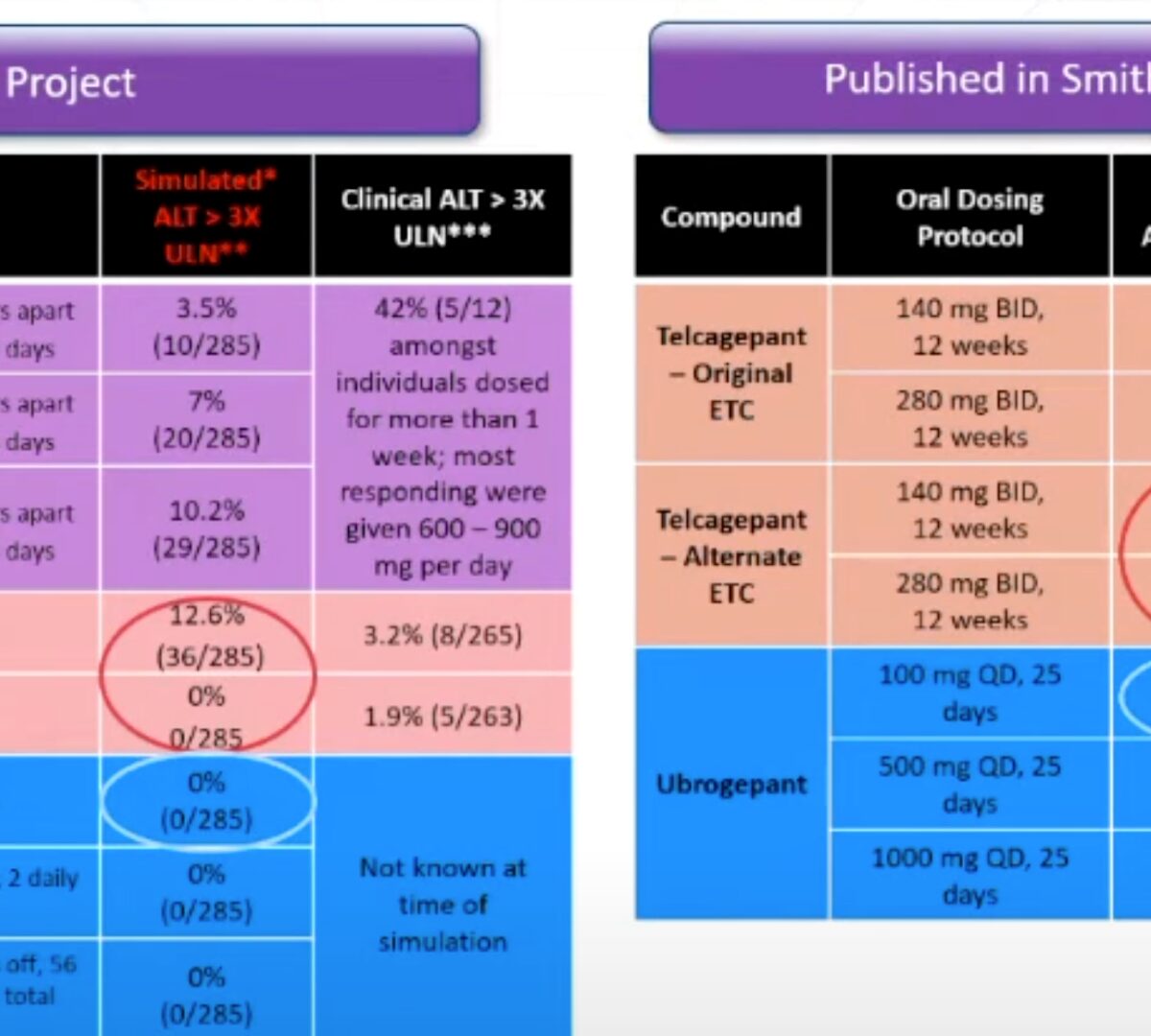
Pre-Clinical Track: Advancing Calcitonin Gene Related Peptide Receptor Antagonists Using QST Modeling to Characterize Next-in-Class Compounds Compared to the Hepatotoxic First in Class Telcagepant
Presented by Jeff Woodhead at SLP MIDD+ Virtual Conference February 16th-17th, 2022
Women in Pharmaceutical Science
Women in Science Session: Ways to Support Women in Science: Representation Matters!
Regulatory Roundtable: Development and validation of PBBM/safe space from the regulatory perspective, a panel discussion
This year we are happy to bring to you a hot topic suggested by last year's attendees. A regulatory roundtable on the Development and validation of PBBM and Safe Space from the regulatory perspective, is a one-hour panel discussion hosted by Sandra Suarez-Sharp and speakers from several regulatory agencies.
Partner Solutions Concierge Program – Taking our Partnership to a Strategic Level
Presented by Josh Fohey at SLP MIDD+ Virtual Conference February 16th-17th, 2022

Pre-Clinical Track: Pushing the Boundaries Beyond – NextGen Session Panel Discussion and Live Q&A
Live Q&A with James Clary, Jeff Woodhead, Lara Clemens, Lisl Shoda, Maxime LeMerdy, Nader Hamzavi, Scott Q. Siler.

Discovery Track: Pushing the Boundaries Beyond – NextGen Session Panel Discussion and Live Q&A
Live Q&A with David Miller, Marvin Waldman, Michael Lawless, Pankaj R. Daga, Phyo Phyo Kyaw Zin, and Robert Fraczkiewicz.
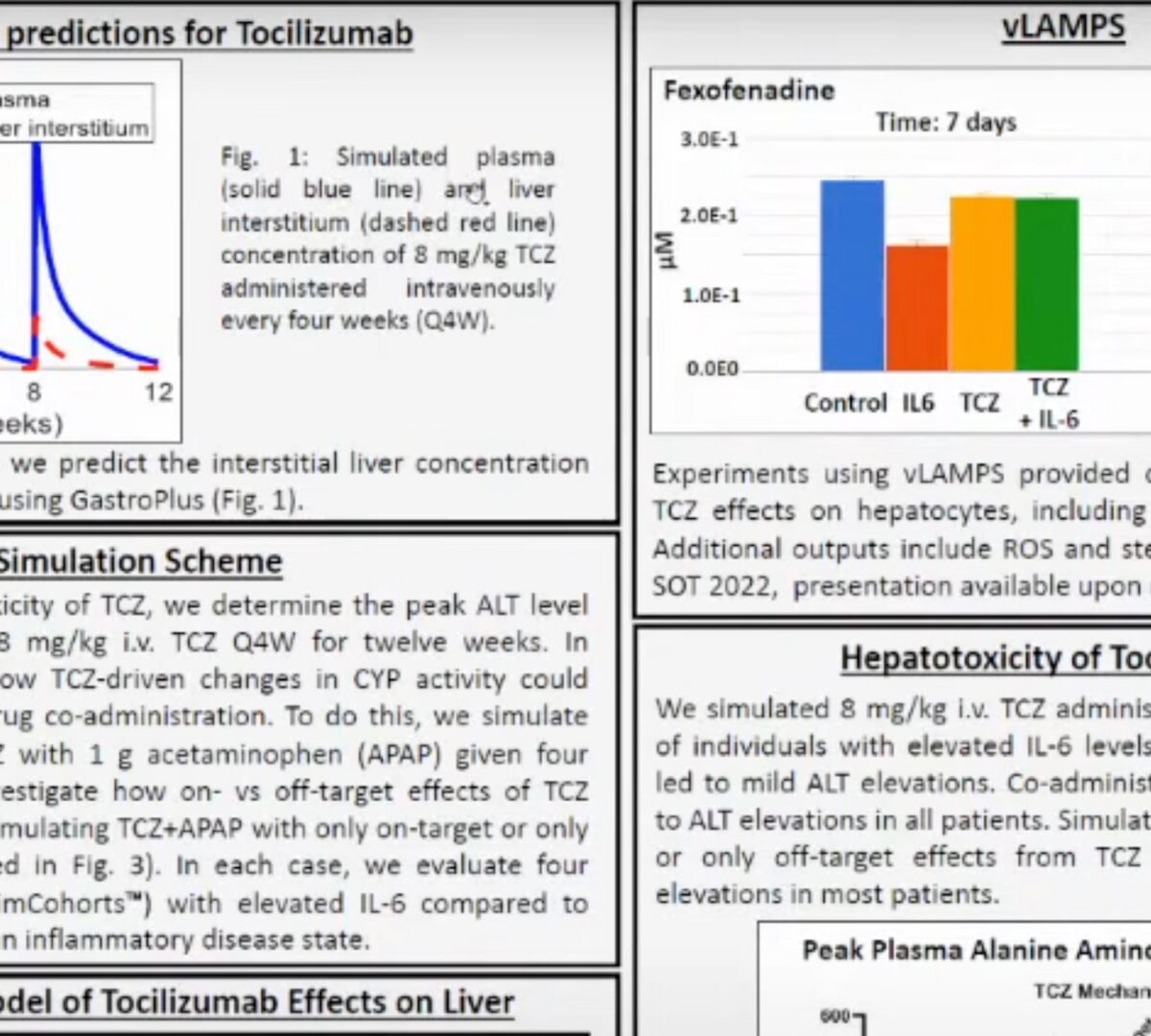
Pre-Clinical Track Poster Presentation: Simulations using BIOLOGXsym demonstrate hepatotoxic potential of Tocilizumab through both on- and off-target effects
Presented by Lara Clemens at SLP MIDD+ Virtual Conference February 16th-17th, 2022
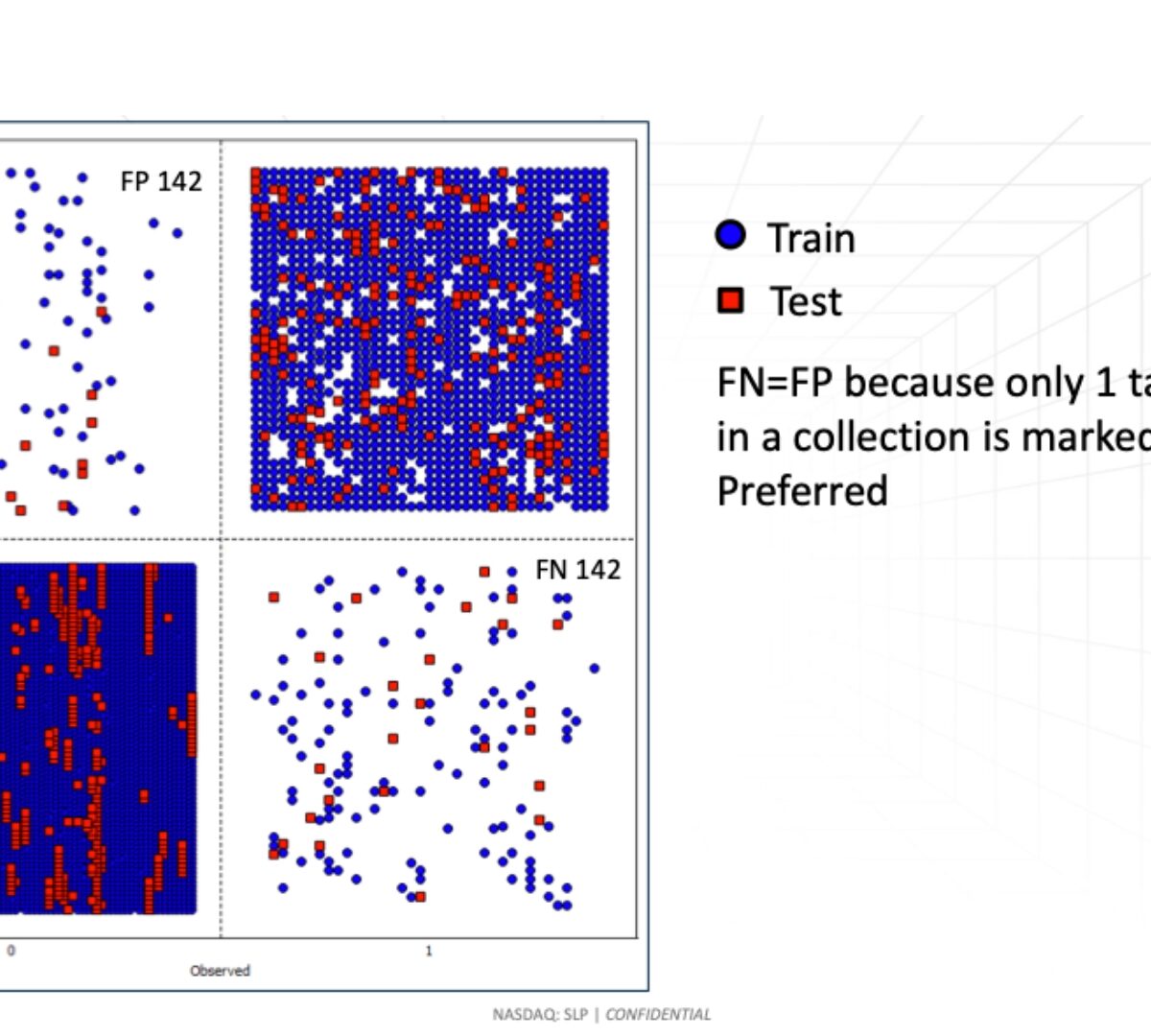
Discovery Track Poster Presentation: Modeling tautomer preference
Presented by Marvin Waldman at SLP MIDD+ Virtual Conference February 16th-17th, 2022

Pre-Clinical Track Poster Presentation: QST Modeling of Drug Induced Acute Proximal Tubule Epithelial Cell Injury and Associated Renal Hemodynamic Responses
Presented by Nader Hamzavi at SLP MIDD+ Virtual Conference February 16th-17th, 2022

Discovery Track: How we leverage automation for data mining, preprocessing & curation
Presented by Phyo Phyo Kyaw Zin at SLP MIDD+ Virtual Conference February 16th-17th, 2022