Researchers found 70% of compounds designed in ADMET Predictor demonstrated significant activity during in vitro testing
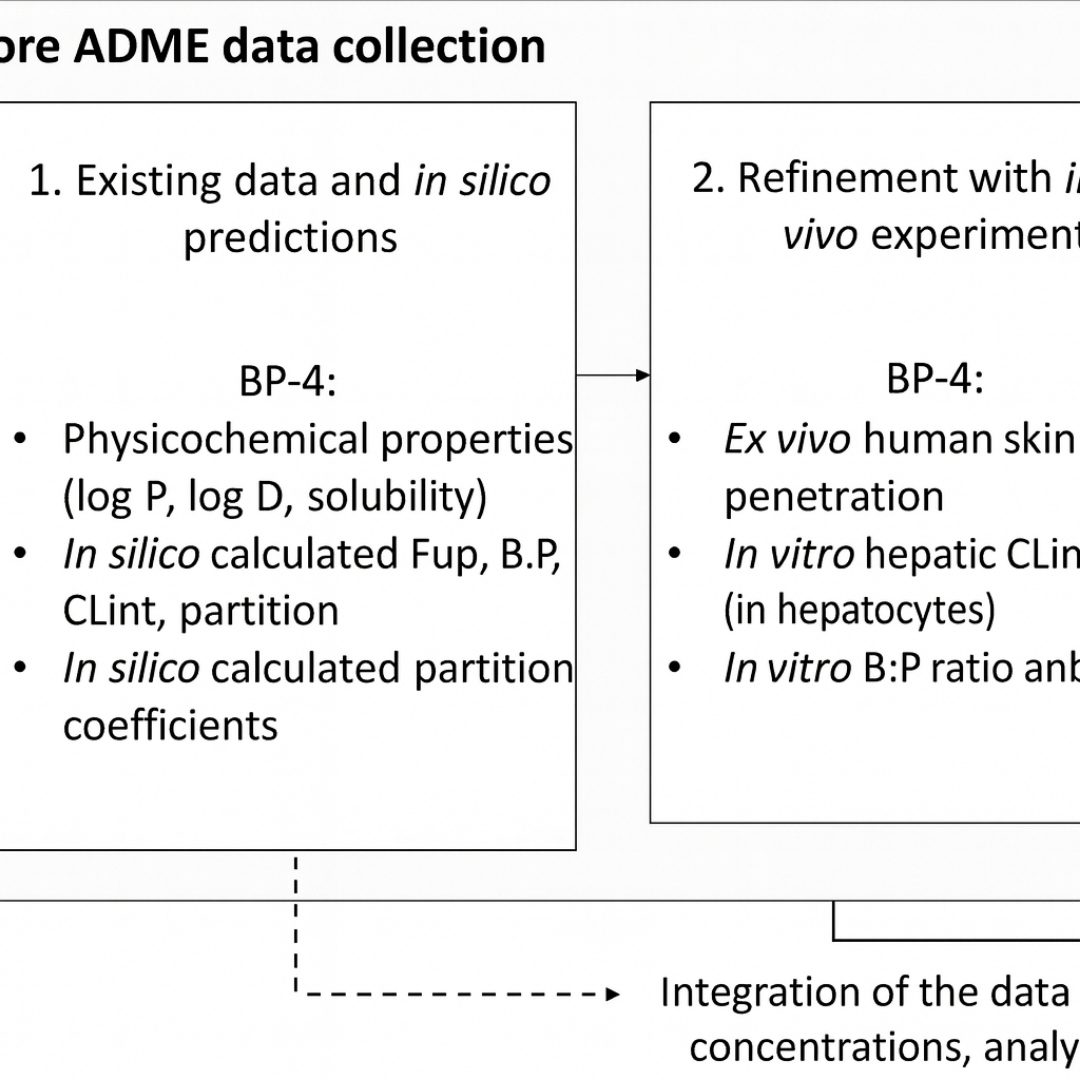
Building Confidence in PBK Model Predictions in the Absence of Human Kinetic Data: Benzophenone-4 Case Study
This study aimed to develop a physiologically based kinetic (PBK) model for benzophenone-4 (BP-4) in humans based on in vitro and in silico input data and to achieve scientific confidence in predicted internal...

Understanding Food Effects in Drug Development: A PBBM Perspective
The co-administration of oral drug products with food can lead to marked alterations in bioavailability (BA) and plasma concentrations when compared to the fasted state.
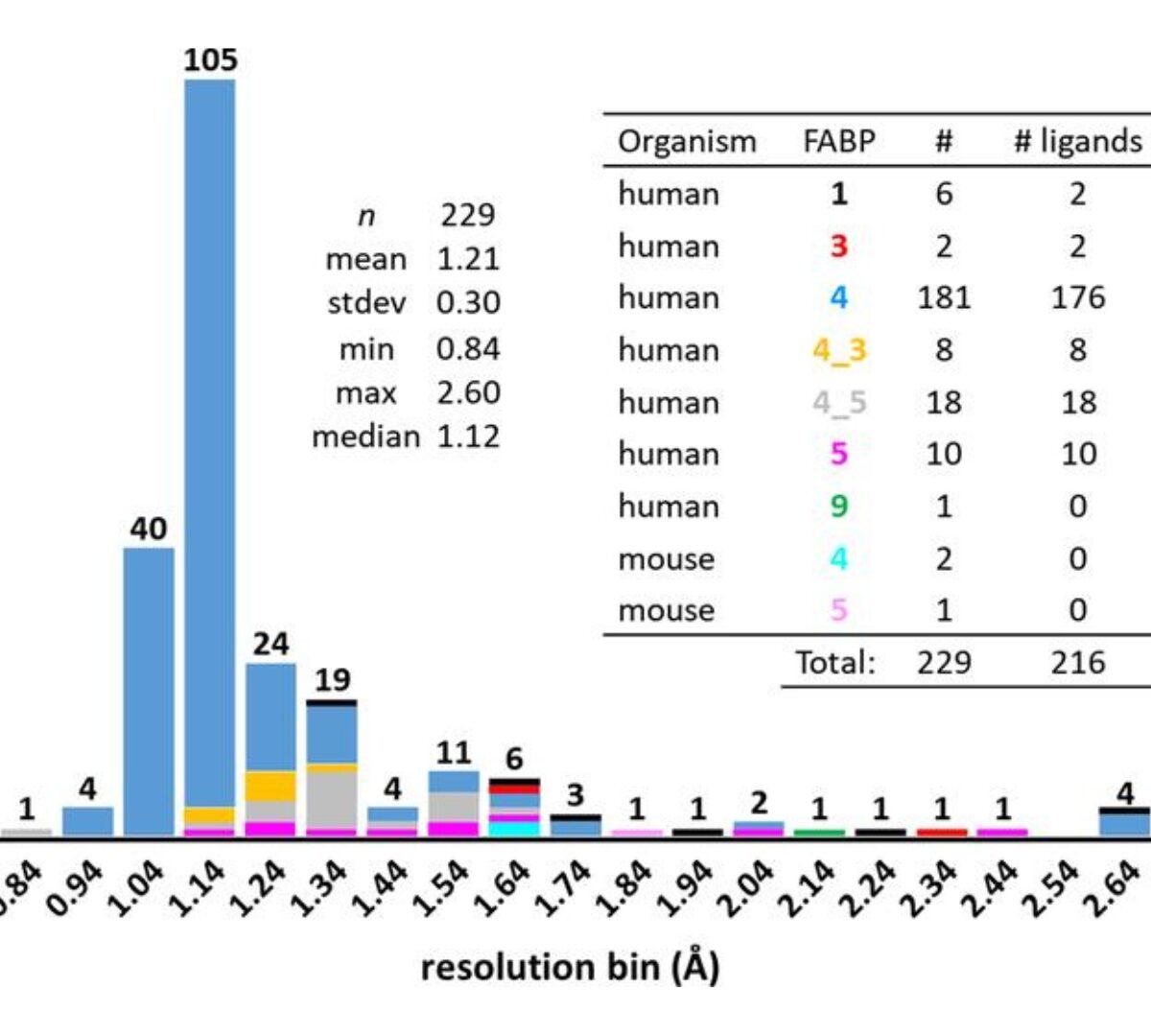
A High-Resolution Data Set of Fatty Acid-Binding Protein Structures. II. Crystallographic Overview, Ligand Classes and Binding Pose
Fatty acid-binding proteins (FABPs) belong to the calycin superfamily of proteins, sharing a similar overall structure with a ten-stranded β-barrel that encloses a large interior cavity for fatty-acid binding.
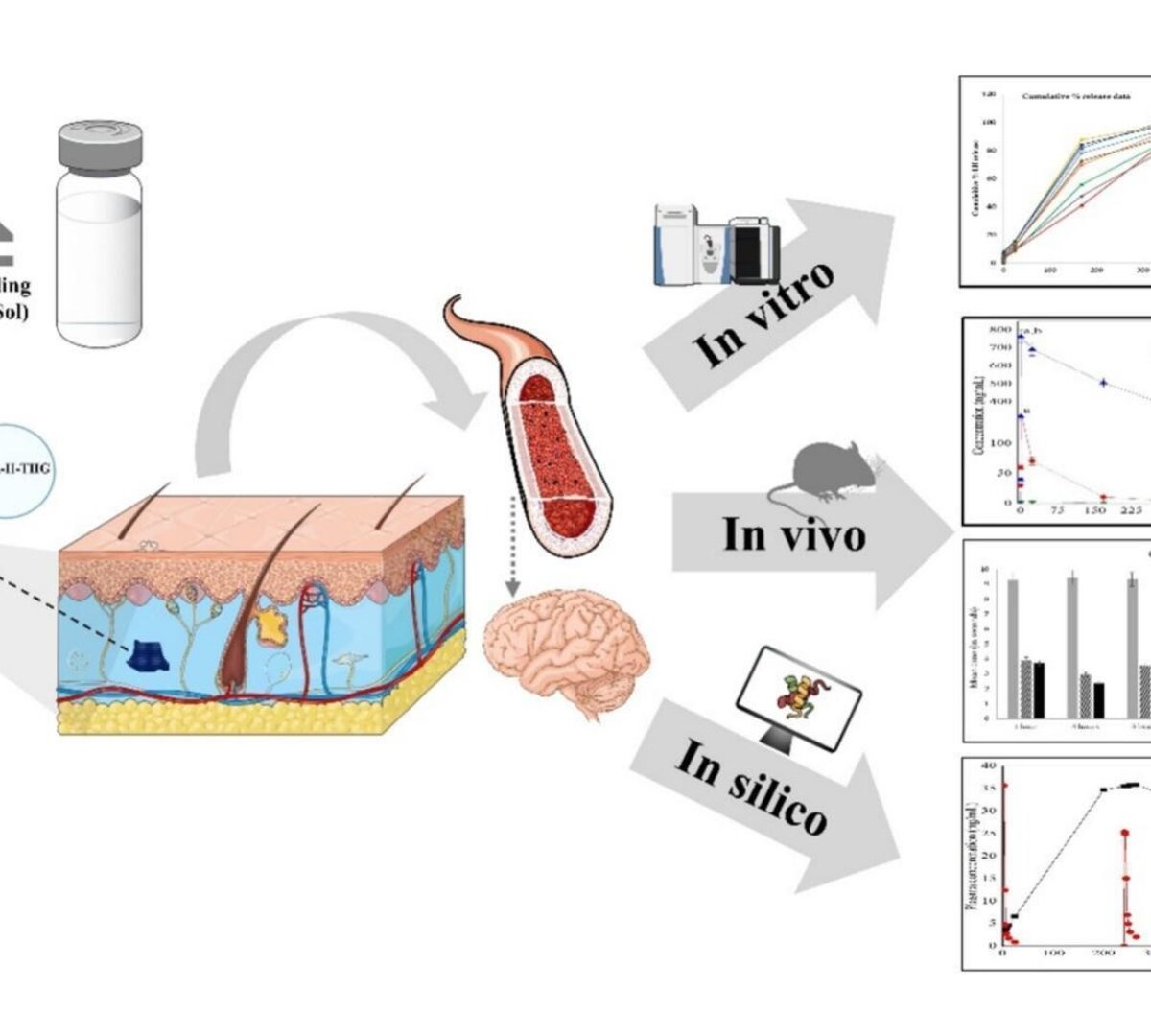
In vitro, in vivo and in silico Assessment of Bioresorbable PLGA-PEG-PLGA Based Thermosensitive Hydrogel Mediated 30-days Delivery of Lurasidone HCl for Schizophrenia
A novel once-monthly sustained-release injectable dosage form of Lurasidone hydrochloride thermosensitive hydrogel (LURA-H-THG) developed using PLGA-PEG-PLGA, for the treatment of schizophrenia.

Privacy Notice (LMS)
Privacy notice for how Simulations Plus handles personal data in its Learning Management System.

DPA Suppliers and Distributors
Simulations Plus data processing agreement for distributors, resellers, and service providers.
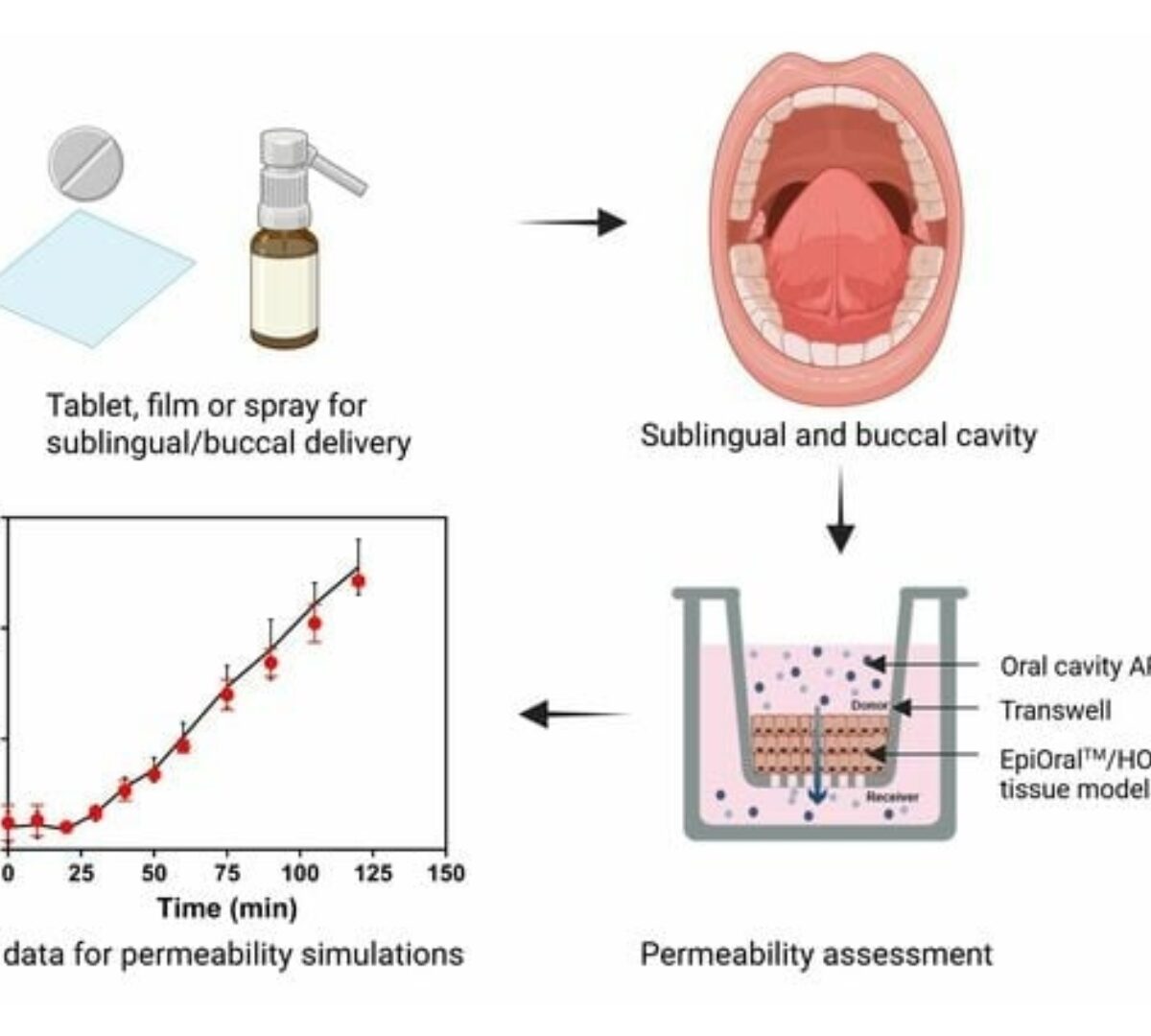
In Vitro Oral Cavity Permeability Assessment to Enable Simulation of Drug Absorption
The oral cavity represents a convenient route of administration for drugs that exhibit significant hepatic first-pass extraction.
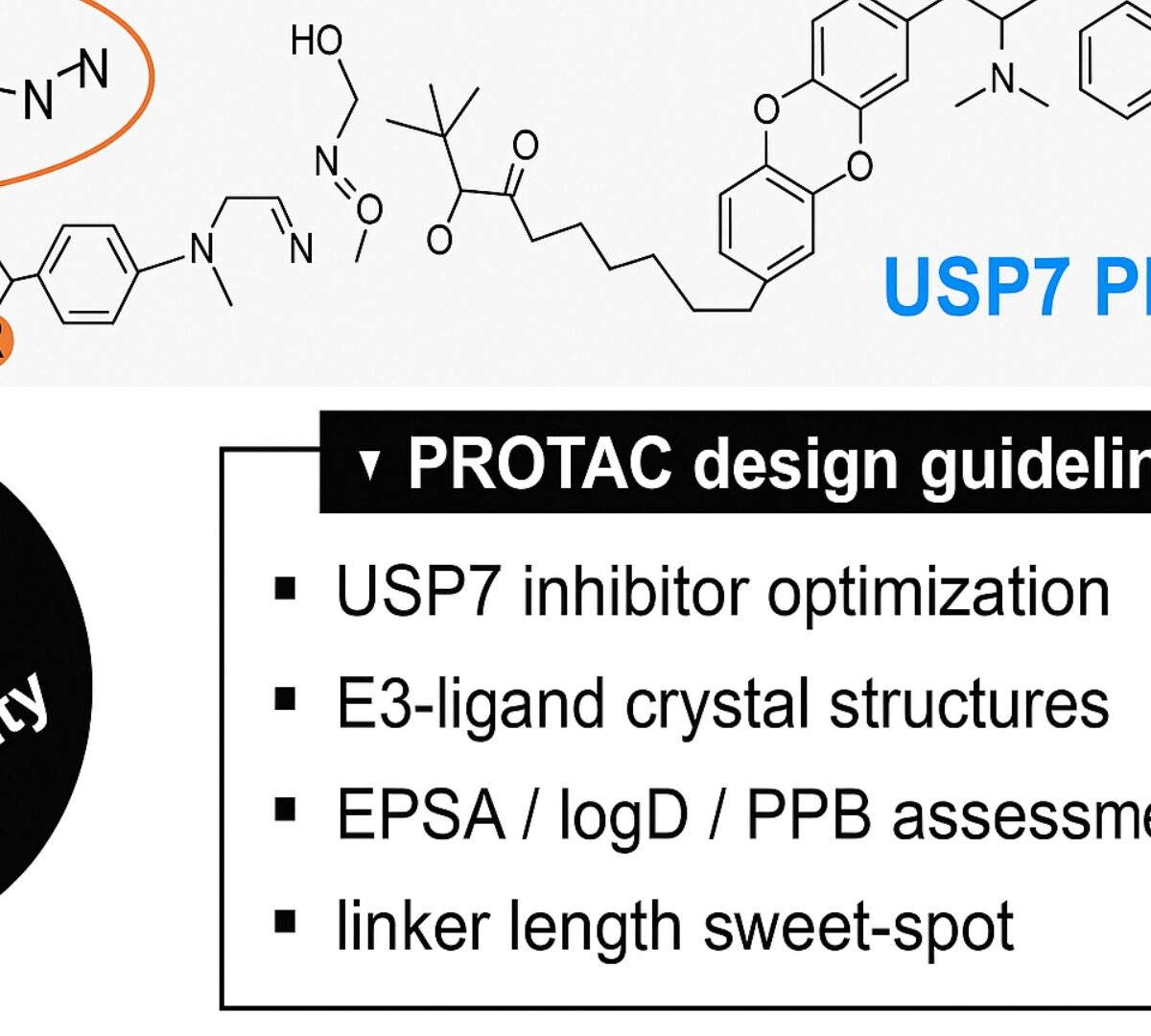
Enhancing Solubility in VHL-Based PROTACs: Optimized USP7 Degraders for Improved Developability
Limited aqueous solubility, high total polar surface area (TPSA), and high hydrogen-bond donor (HBD) counts have hampered the clinical development of VHL-based proteolysis-targeting chimeras (PROTACs).
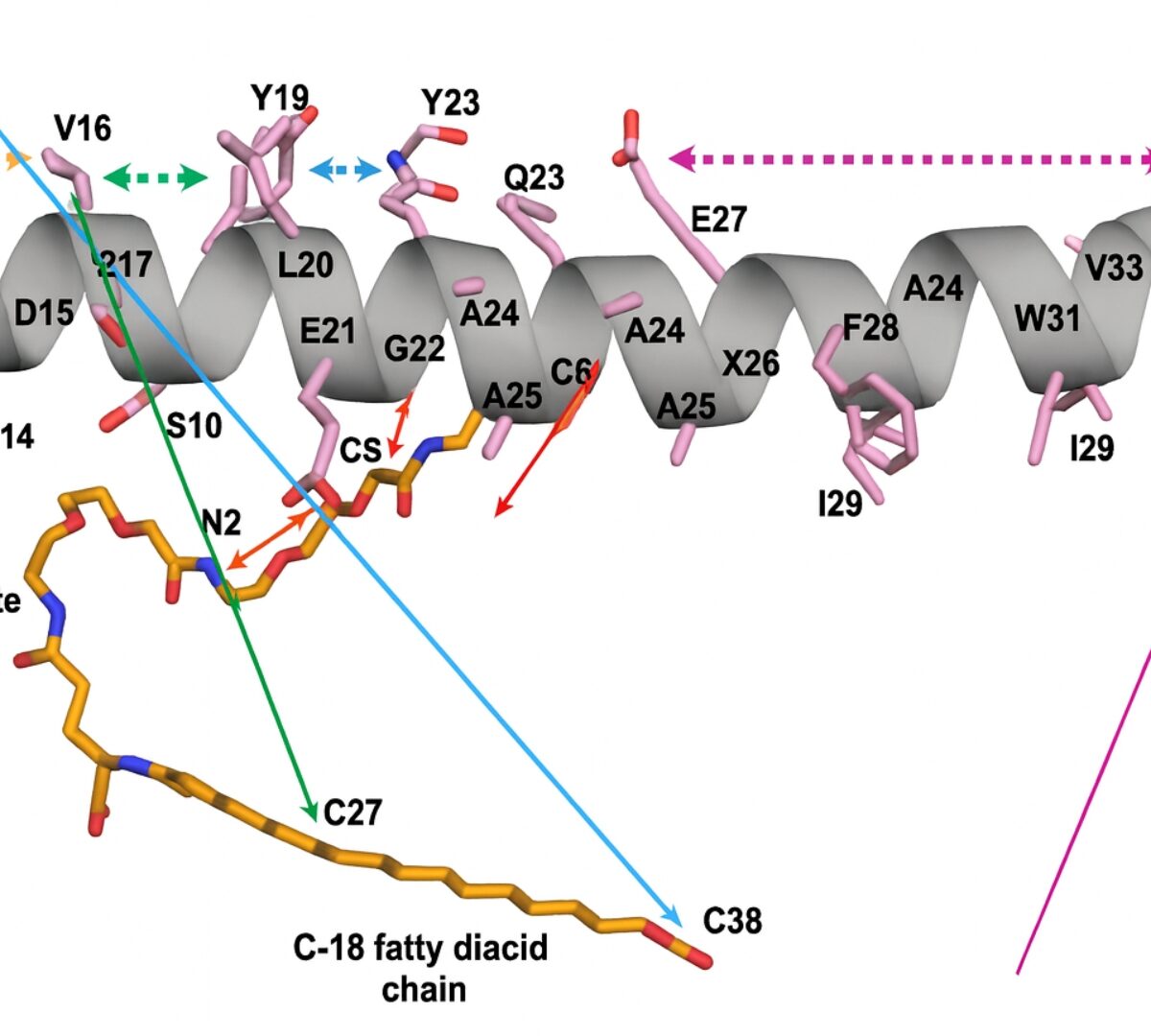
Oral Absorption of Semaglutide: Pharmacokinetic Modeling and Molecular Dynamics Simulations
Semaglutide is a GLP-1 receptor agonist that is formulated for oral administration as Rybelsus®.

Simulations Plus Invests in Clinical Development Technology Company Nurocor
Investment to accelerate innovations in biopharma development through Corporate Development Initiative

Simulations Plus Reports Third Quarter Fiscal 2025 Financial Results
Updated full-year revenue guidance of between $76 to $80 million and adjusted diluted EPS of $0.93 to $1.06

Physiologically Based Pharmacokinetic Modeling and Mechanistic In Vitro-In Vivo Correlation for Long-Acting Injectable Suspension
Long acting injectable (LAI) suspensions demonstrate extended release by forming depots at the injection site (subcutaneous or intramuscular), from which poorly soluble drugs are slowly dissolved and absorbed into the systemic circulation
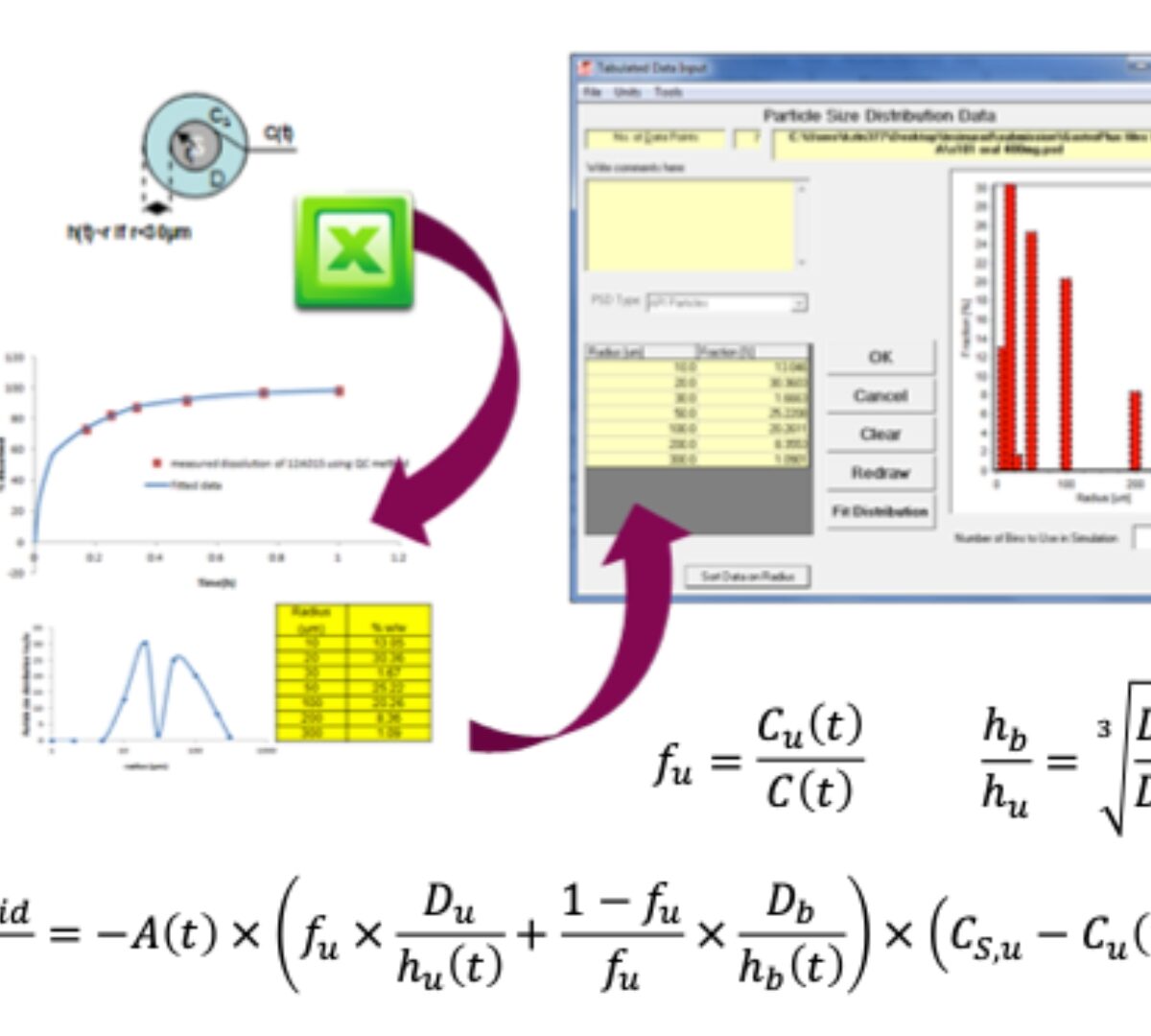
When Mechanistic IVIVC Falls Short: The Case for IVIVR in Oral Drug Delivery
Traditional IVIVC is robust for ER formulations where dissolution is rate limiting.
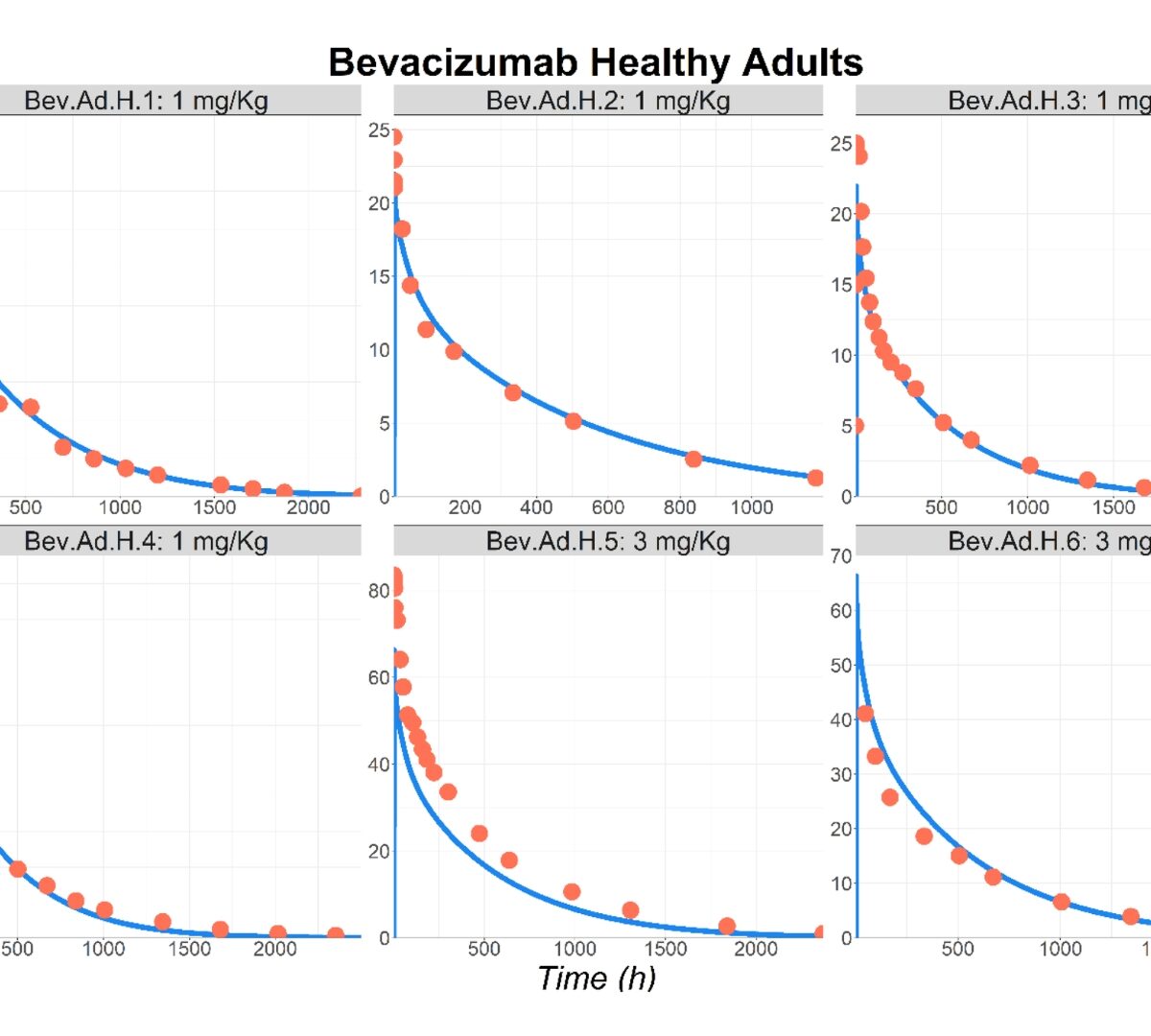
Prediction of Monoclonal Antibody Pharmacokinetics in Pediatric Populations Using PBPK Modeling and Simulation
Accurately determining pediatric dosing is essential prior to initiating clinical trials or administering medications in routine clinical settings.
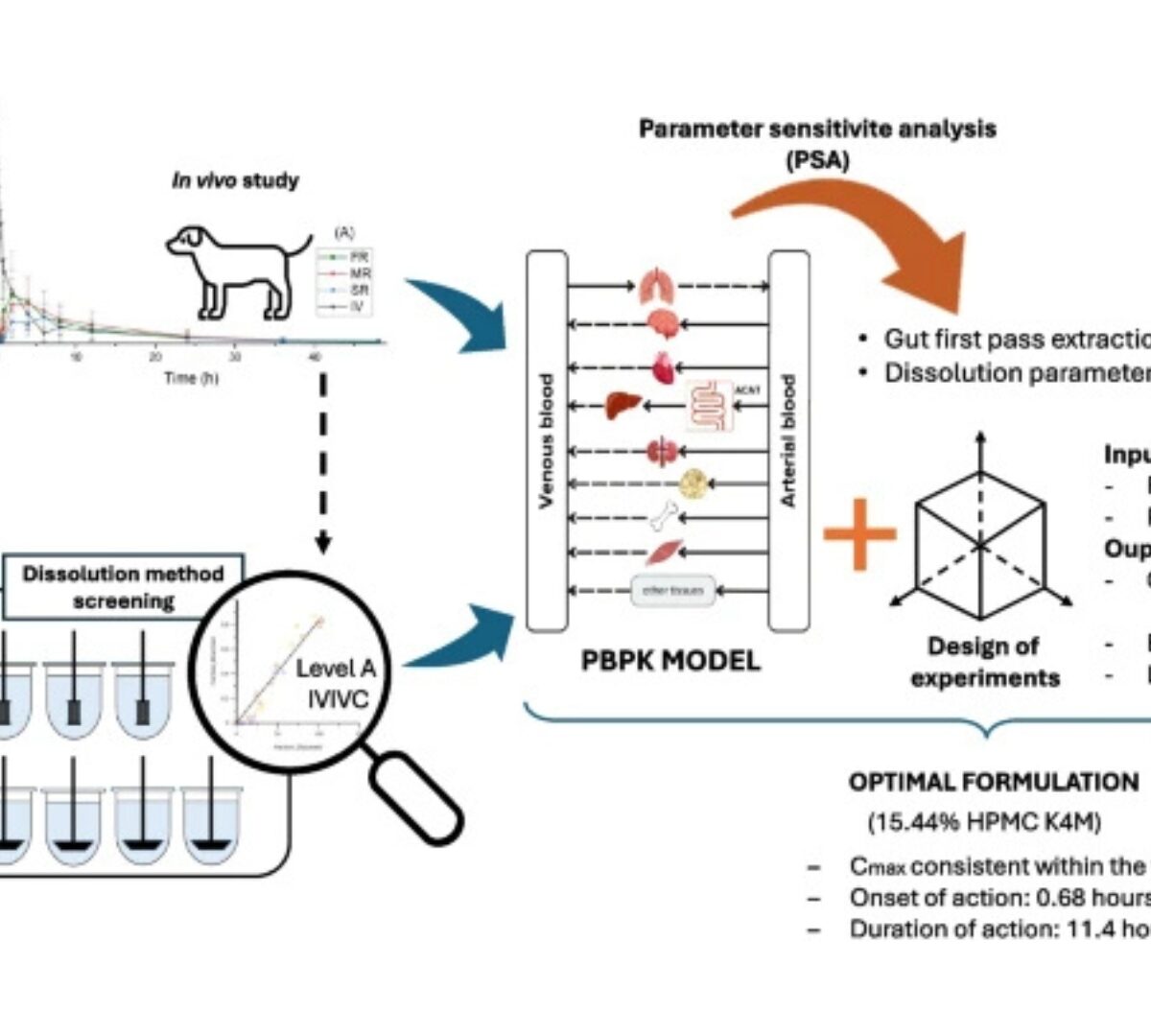
Optimizing Extended-release Formulation of l-tetrahydropalmatine Based on In Vivo Outcomes Using Integrated Modeling Approaches
l-Tetrahydropalmatine (l-THP) is a promising drug candidate for addiction treatment and needs to be delivered in extended-release dosage forms for safety and efficiency.
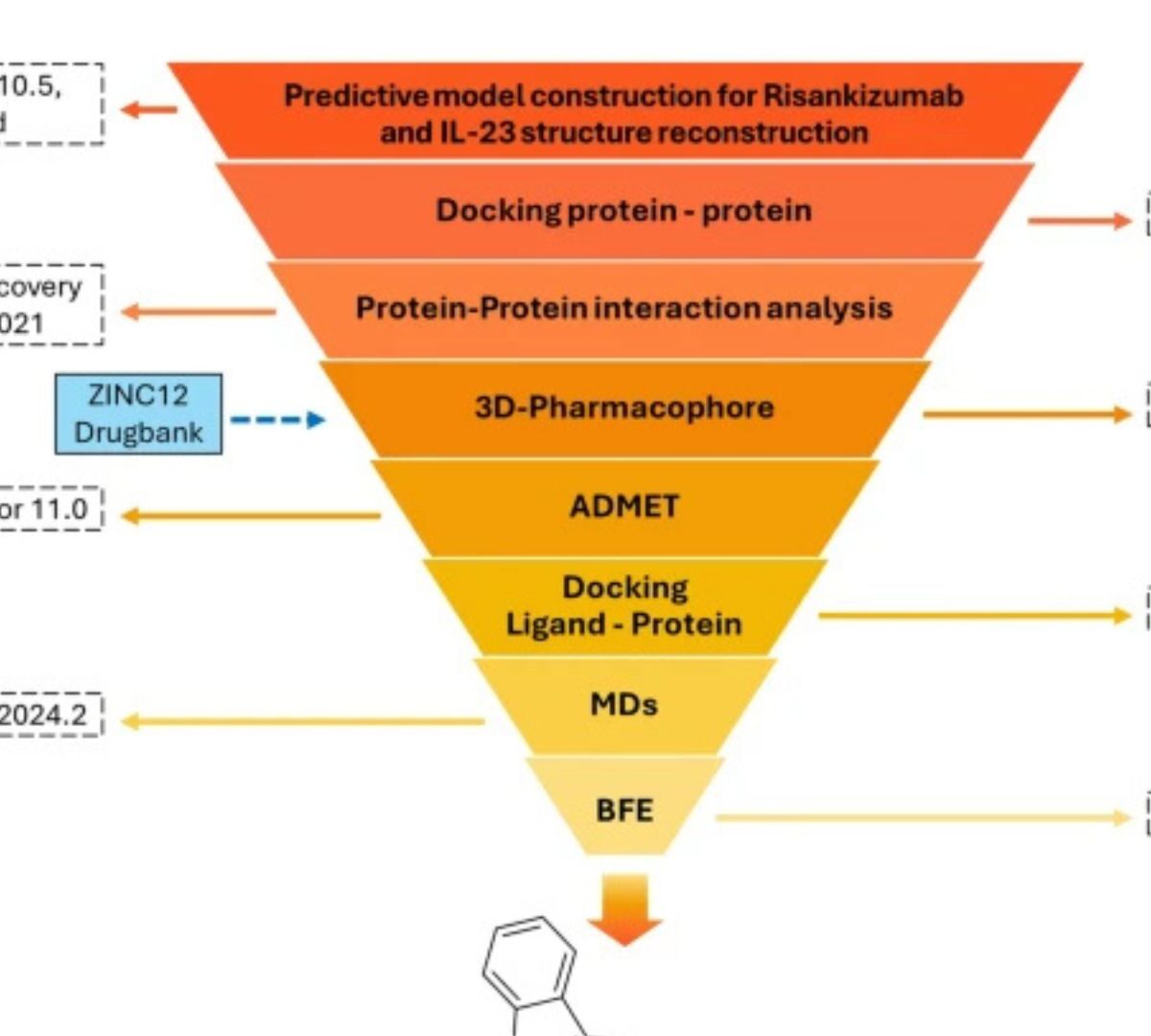
Structure-Based Screening of Small-Molecule Interleukin-23 Inhibitors Inspired by Monoclonal Antibody Interactions
Interleukin-23 (IL-23) is a key driver of chronic inflammatory diseases, yet current therapies rely on costly monoclonal antibodies.

Thriving in the GenAI Era: A Guide for Scientists
In systems biology, we often speak of emergence—how complex systems yield behaviors not apparent from their individual parts.

Simulations Plus Announces Preliminary Third Quarter Fiscal 2025 Revenue
Updates fiscal 2025 revenue guidance range
Third quarter fiscal 2025 full results to be released on July 2, 2025, with conference call at 5 p.m. ET
