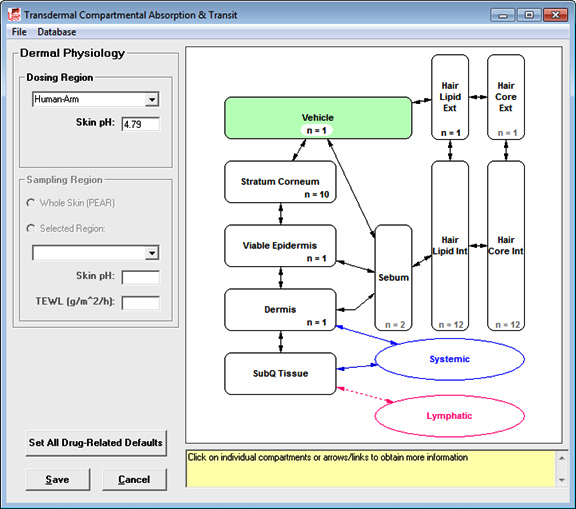
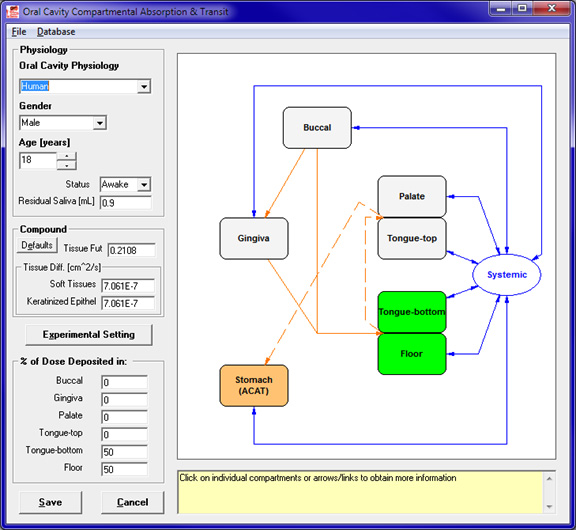
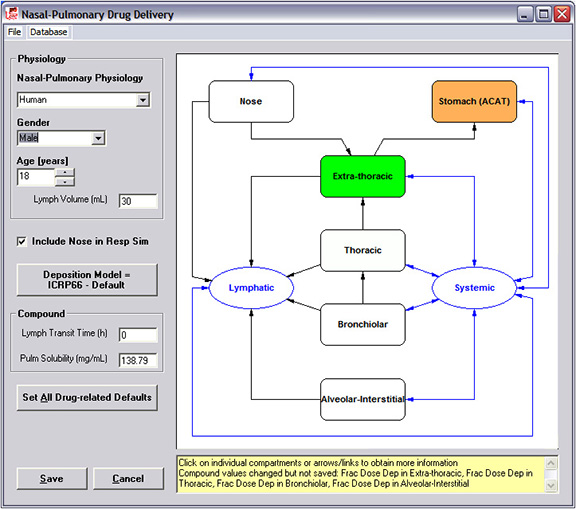
The Additional Dosage Routes Module in GastroPlus extends the program beyond the traditional oral and intravenous drug administration routes. With this module, you can simulate mechanistic absorption and disposition through dermal (topical and subcutaneous), intraoral (oral cavity), pulmonary (respiratory), ocular, intramuscular, and intraarticular routes. The long-acting injectable models can be utilized for subcutaneous, intramuscular, and NEW! intraarticular routes. These models were all developed in collaboration with the top 5 pharmaceutical companies and/or the U.S. FDA. The ability to predict concentration profiles in different regions of the skin, mouth, eye, lungs, nose, and muscle can help you:
- Explore various formulation/drug delivery options to achieve desired therapeutic effects
- Identify species-specific changes to estimate how a drug is handled in animals vs. humans