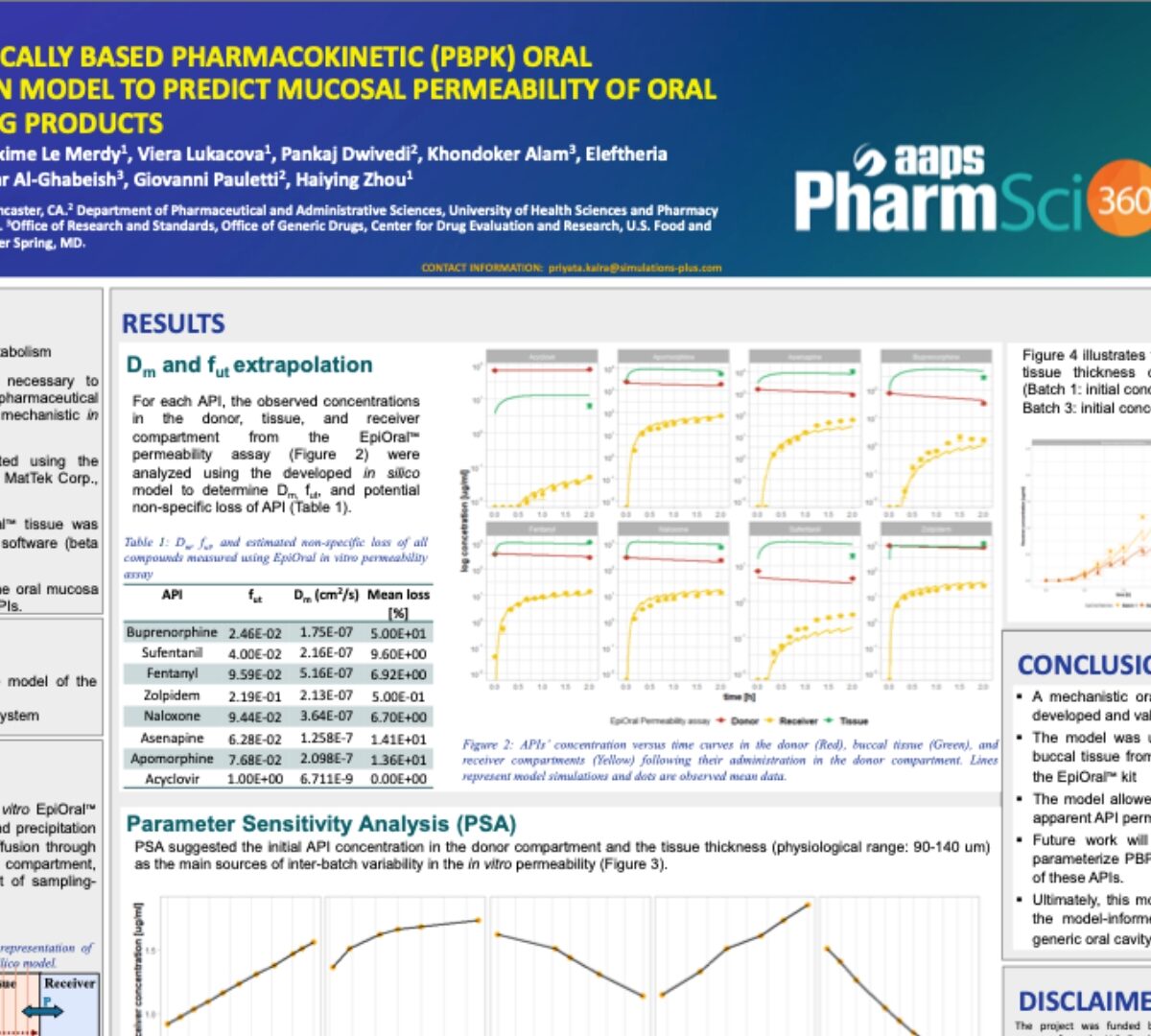
Buccal delivery allows bypassing first-pass metabolism
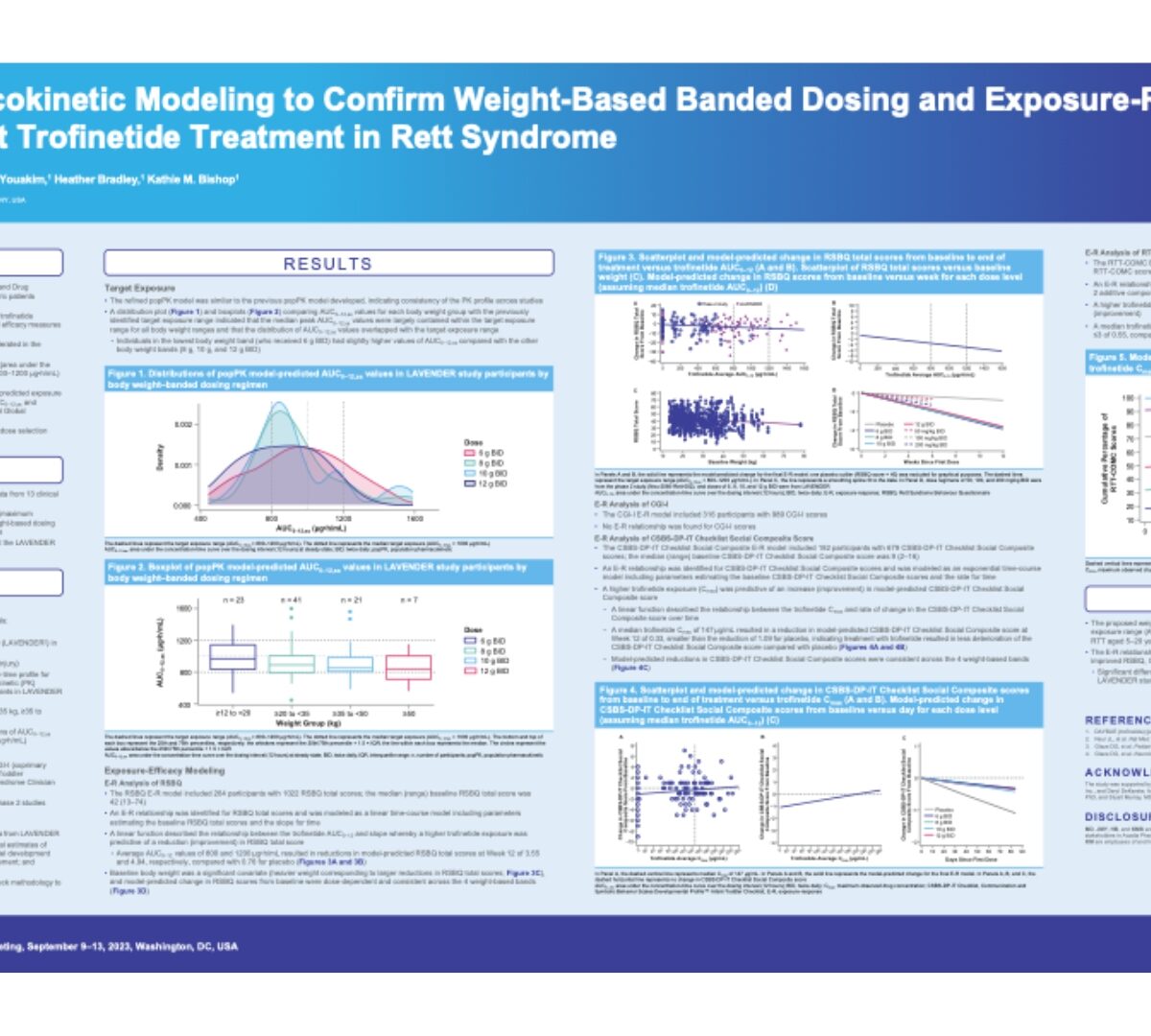
Population pharmacokinetic modelling to confirm weight-based banded dosing and exposure-response efficacy analyses to support trofinetide treatment in rett syndrome
Trofinetide, a synthetic analog of glycine-proline-glutamate, was approved by the US Food and Drug Administration in March 2023 for the treatment of...
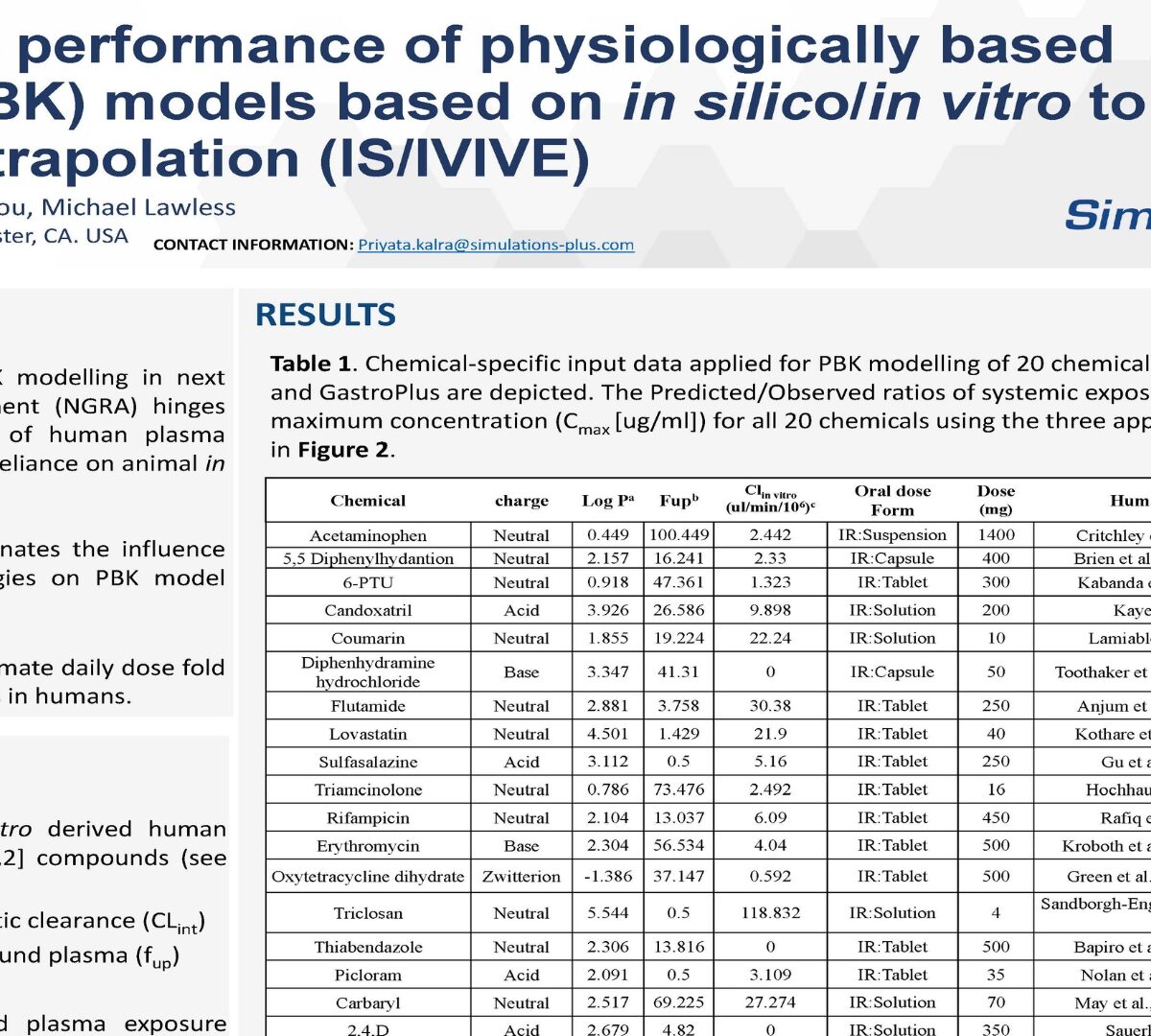
Predictive performance of physiologically based kinetic (PBK) models based on in silic o/ in vitro to in vivo extrapolation (IS/IVIVE)
The applicability of PBK modelling in next generation risk assessment ( hinges on accurate prediction of human plasma concentrations without reliance on animal in vivo kinetics data.
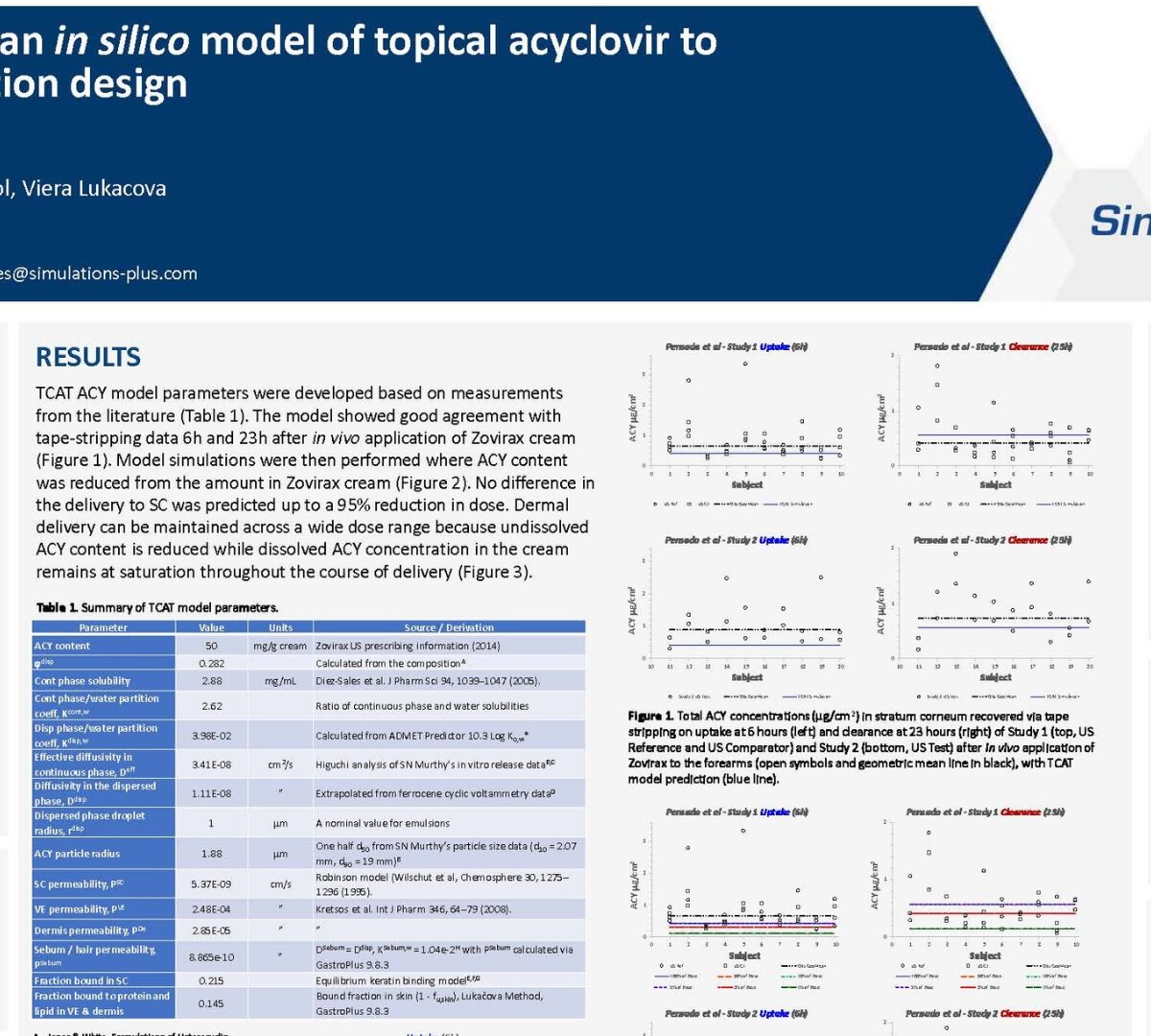
Development of an in silico model of topical acyclovir to explore formulation design
Acyclovir (ACY) creams are used for local treatment of HSV-1 infections in the basal epidermis.
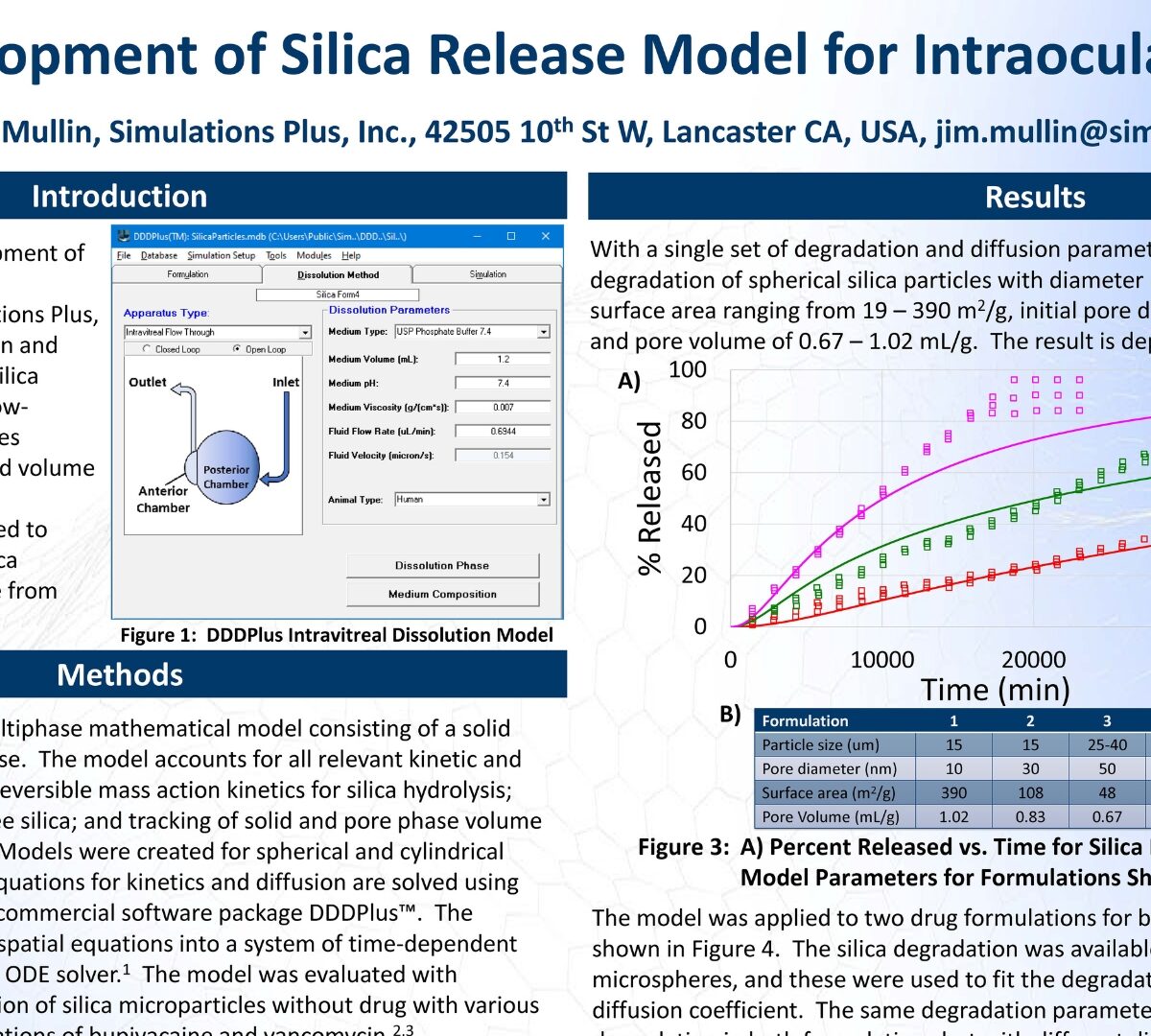
Development of Silica Release Model for Intraocular Injections
This poster reviews the development of a numerical framework within DDDPlus™ (Version 6.0, Simulations Plus, Inc.) to simulate the degradation and drug...
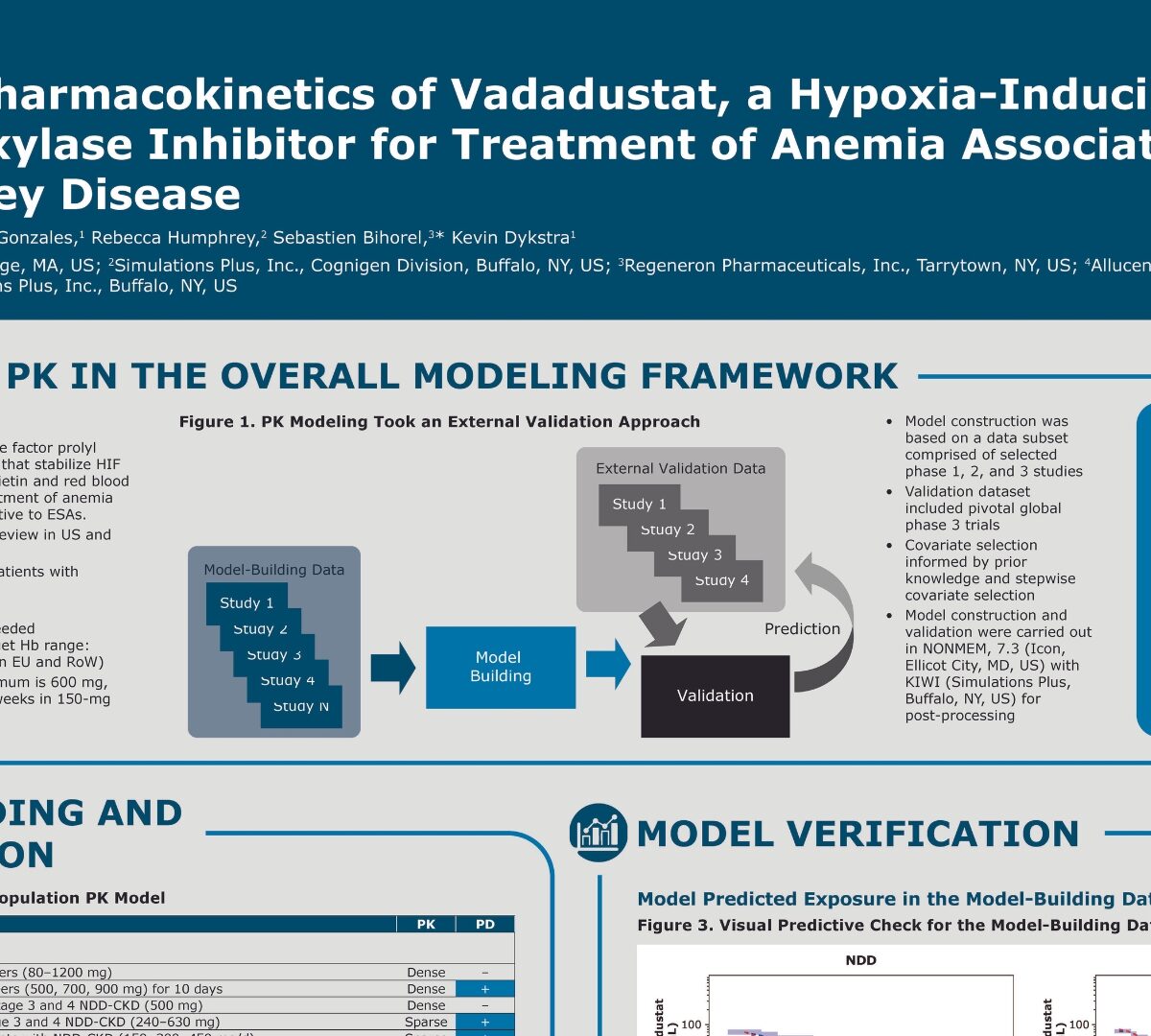
Population pharmacokinetics of vadadustat, a hypoxia-inducible factor prolyl hydroxylase inhibitor for treatment of anemia associated with chronic kidney disease
Vadadustat is an oral hypoxia-inducible factor prolyl hydroxylase inhibitor, a class of...
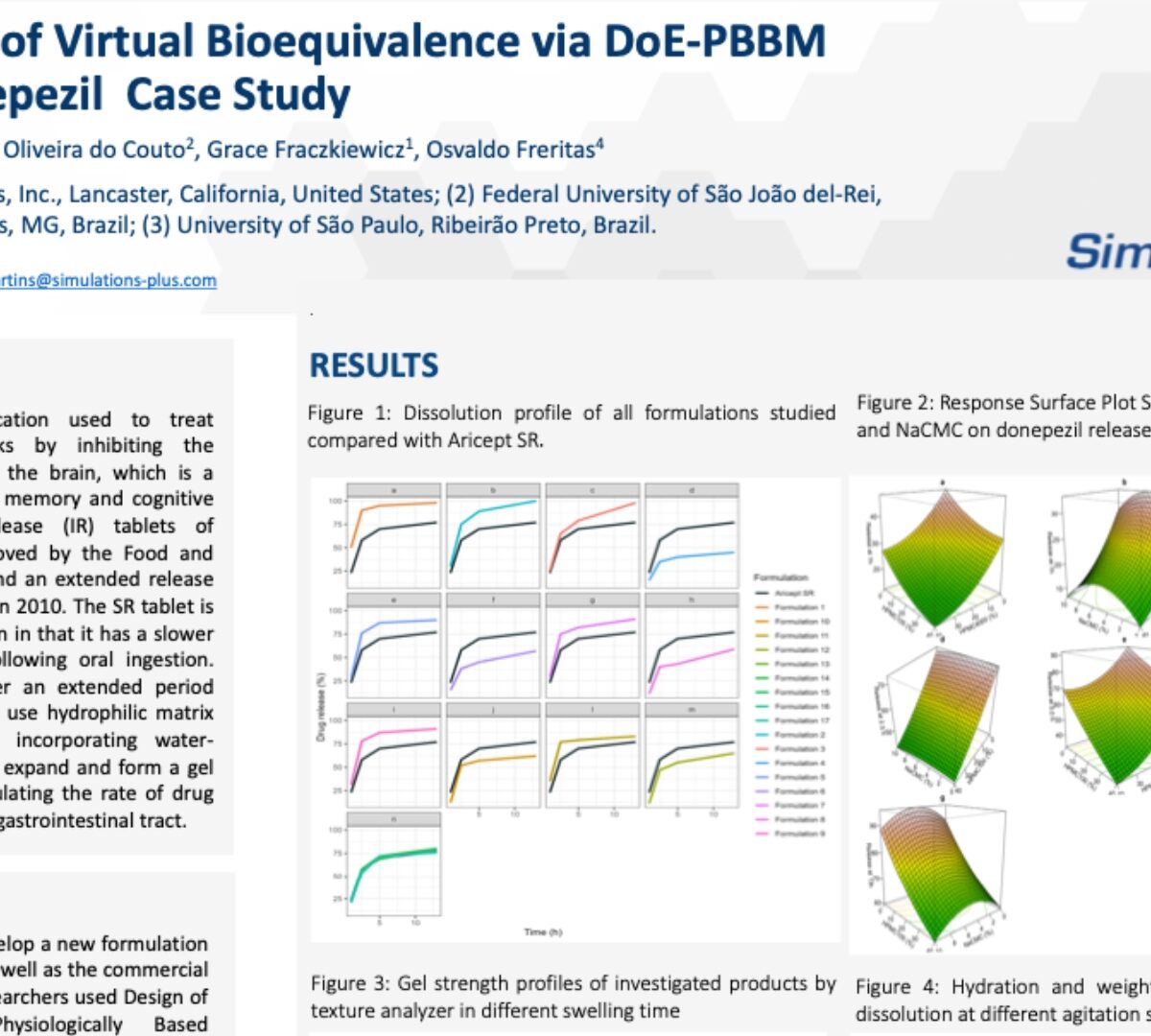
Establishment of Virtual Bioequivalence via DoE-PBBM Model: A Donepezil Case Study
Donepezil (DZP) is a medication used to treat Alzheimer's disease.

The application of AI-driven Drug Discovery technology for molecular optimization of nuclear receptor ligands
Nuclear receptors (NRs) are a superfamily of transcription factors whose activity is regulated upon the binding of a specific ligand.
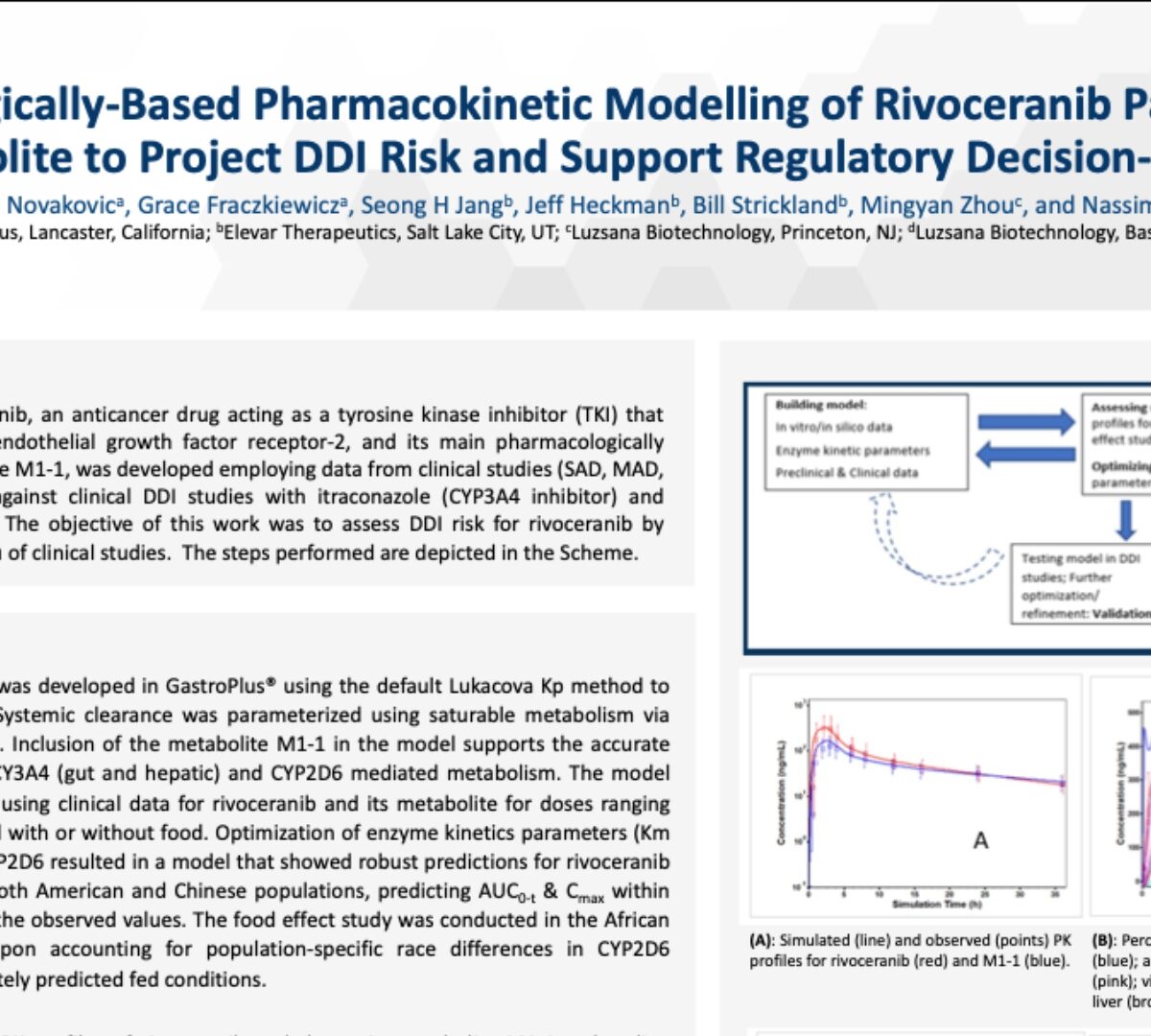
Physiologically-Based Pharmacokinetic Modelling of Rivoceranib Parent and Metabolite to Project DDI Risk and Support Regulatory Decision-Making
The PBPK model for rivoceranib, an anticancer drug acting as a tyrosine kinase inhibitor (TKI) that selectively targets vascular...
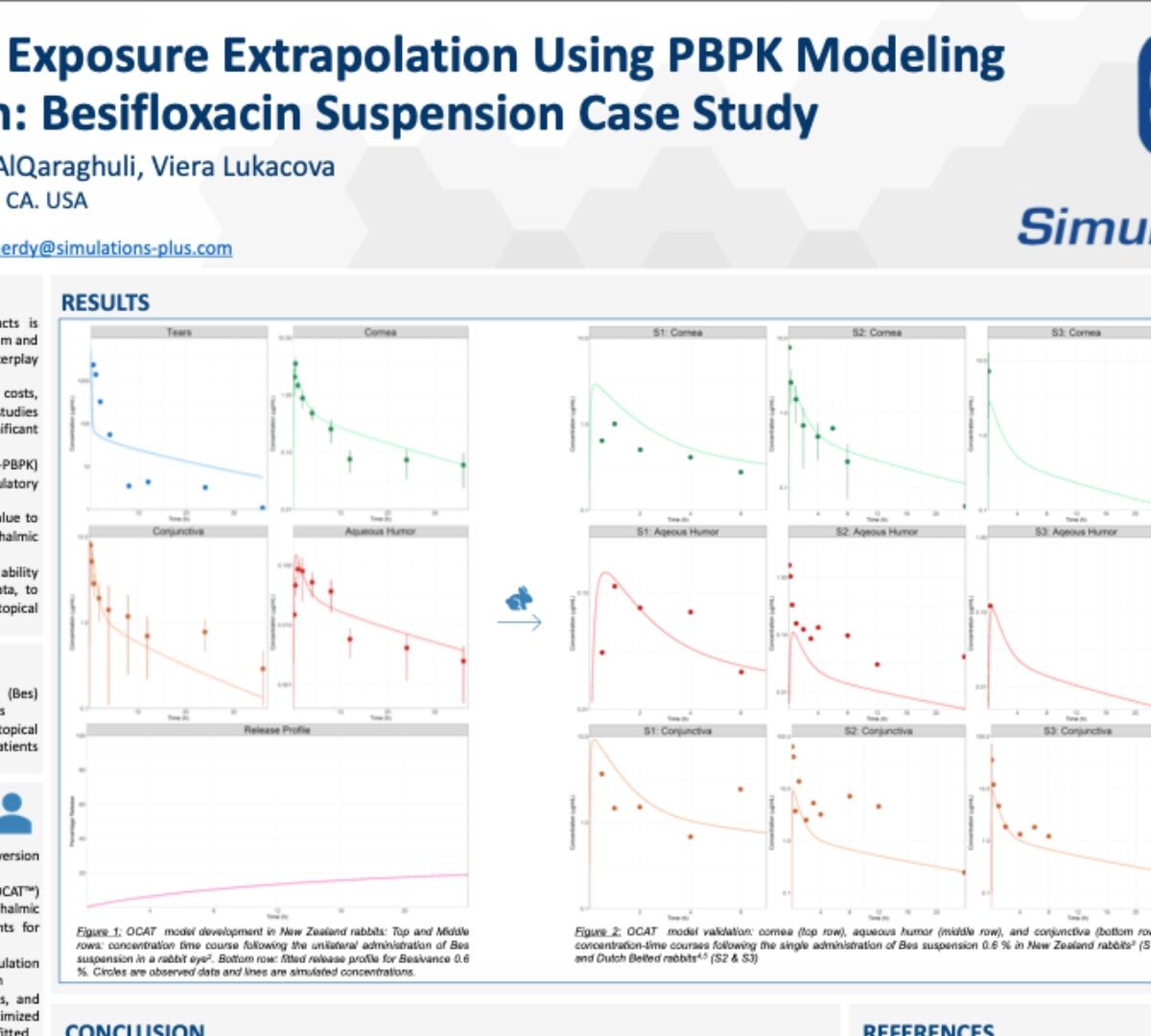
Clinical Ocular Exposure Extrapolation Using PBPK Modeling and Simulation: Besifloxacin Suspension Case Study
The purpose of this research is to demonstrate the ability of O-PBPK models, validated against rabbit PK data, to predict clinical ocular exposure, following topical administration of ophthalmic suspensions
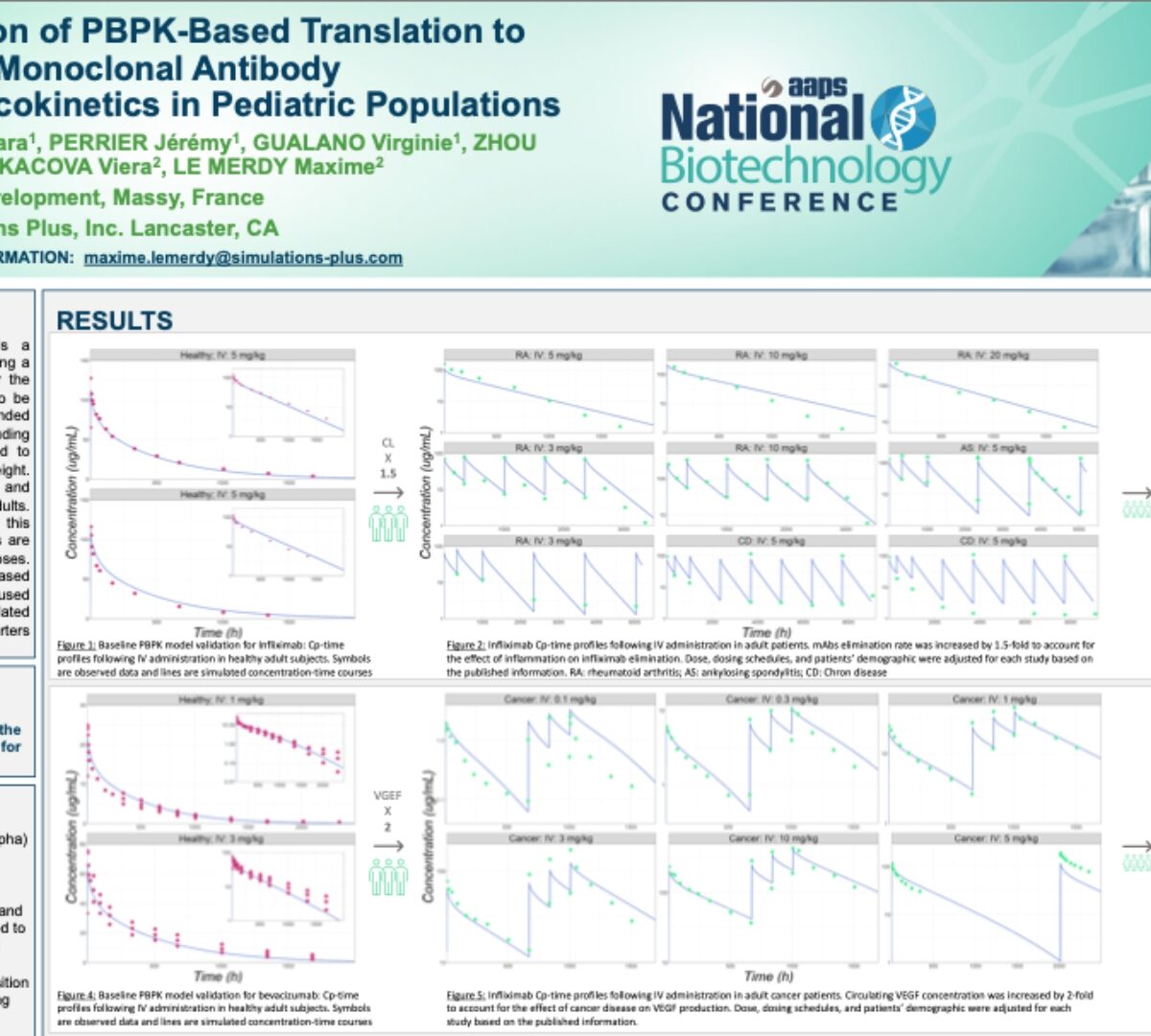
Validation of PBPK-Based Translation to Predict Monoclonal Antibody Pharmacokinetics in Pediatric Populations
Accurate prediction of the pediatric dose is a necessity before conducting a clinical trial or using a drug product in standard clinical practices.
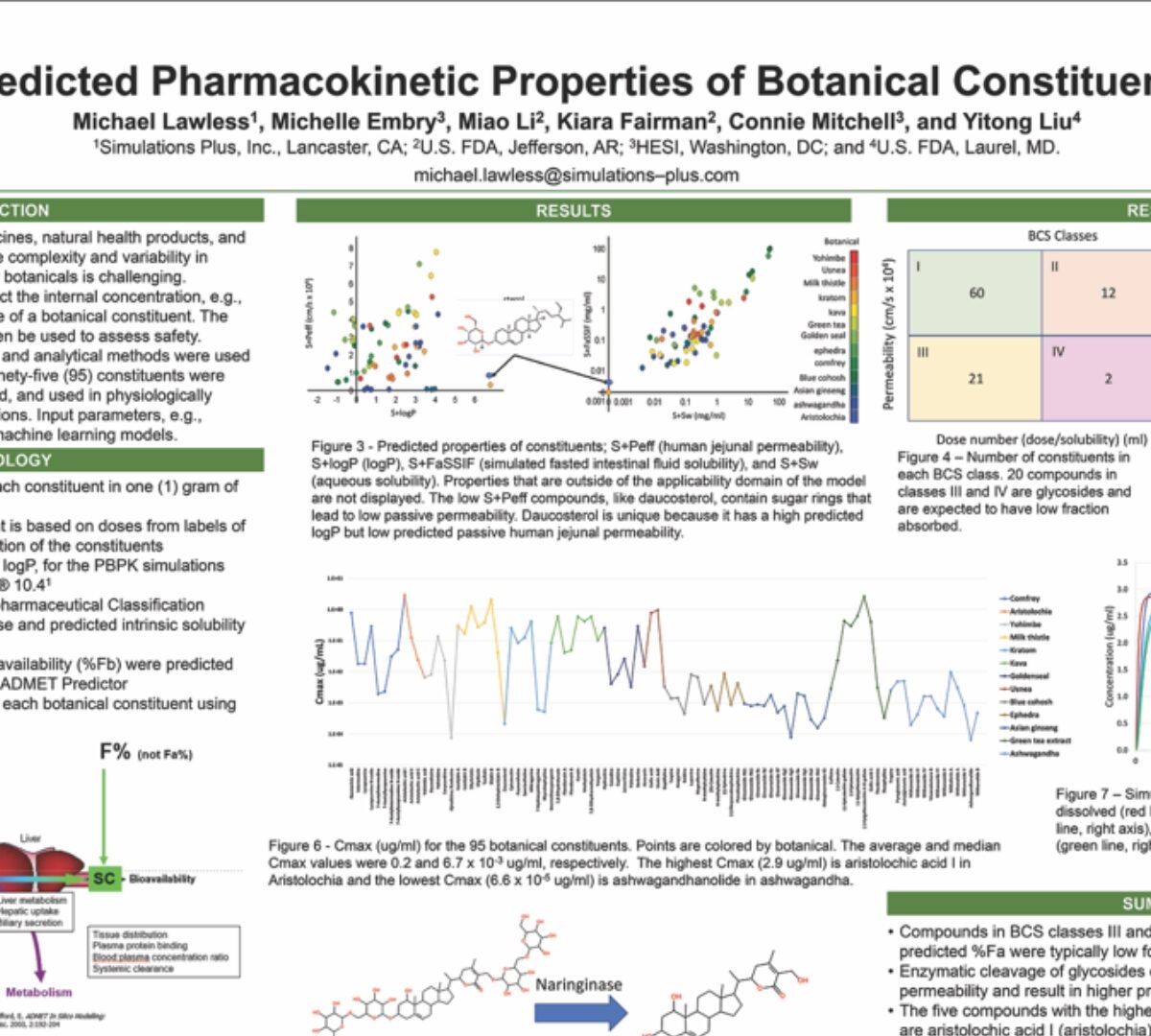
Predicted Pharmacokinetic Properties of Botanical Constituents
Botanicals are used as traditional medicines, natural health products, and dietary supplements globally.
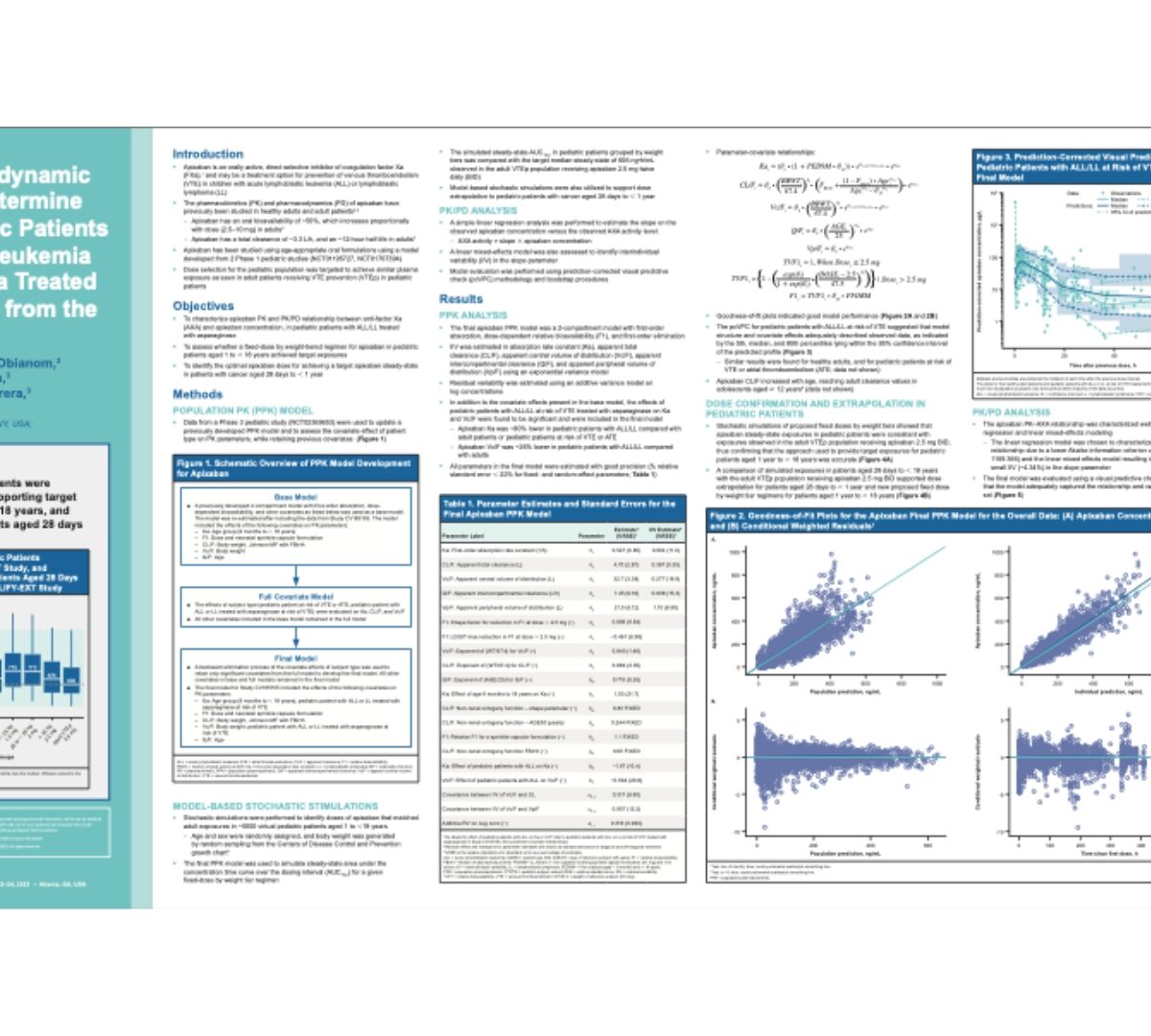
Pharmacokinetic/pharmacodynamic analysis of apixaban to determine dosing regimens for pediatric patients with acute lymphoblastic leukemia or lymphoblastic lymphoma treated with asparaginase: analysis from the PREVAPIX study
Apixaban is an orally active, direct selective inhibitor of coagulation factor Xa (FXa),1 and may be a treatment option for prevention of venous thromboembolism (VTE) in children with...
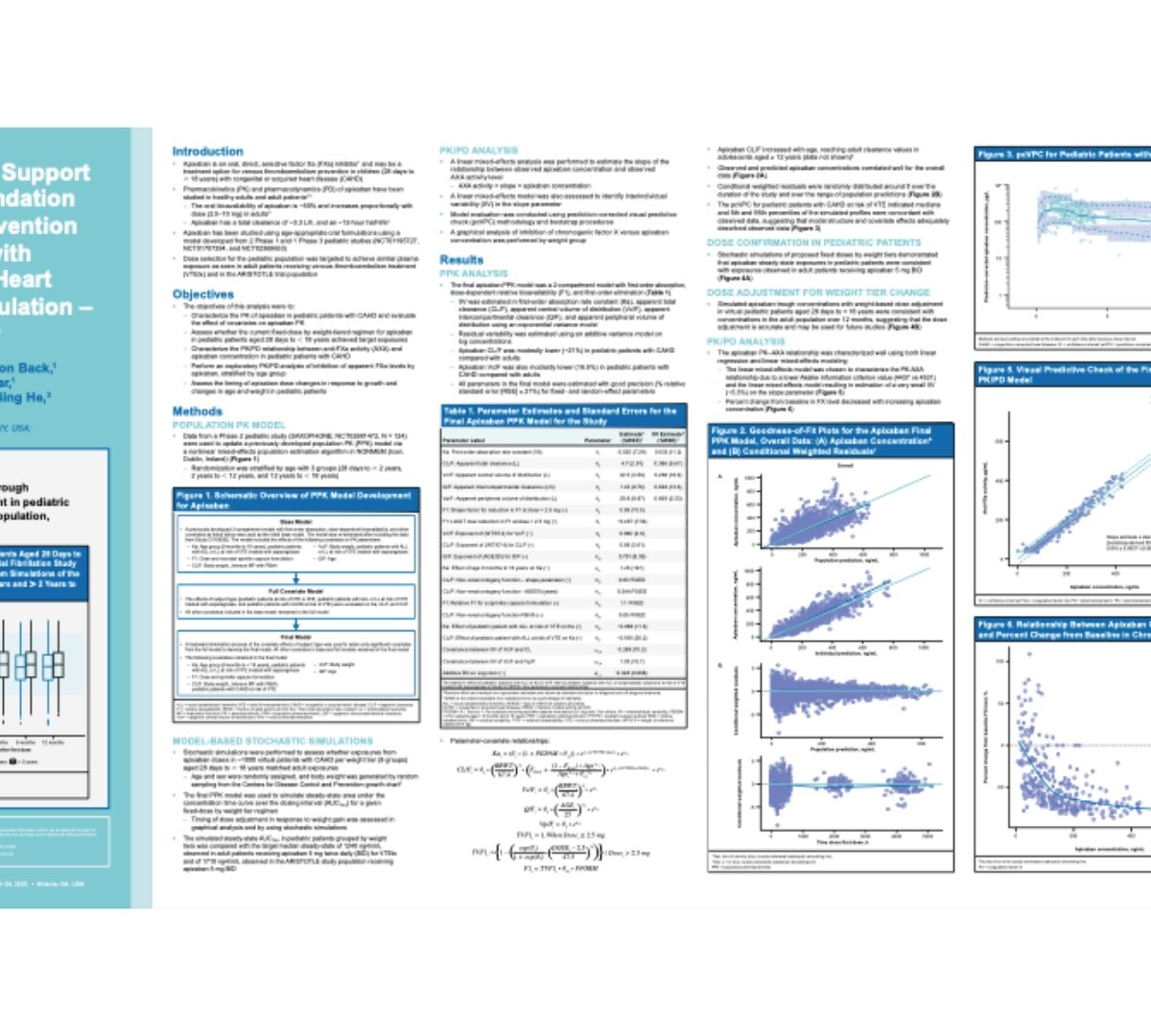
Modeling and simulation to support apixaban dose recommendation for thromboembolism prevention in pediatric subjects with congenital or acquired heart disease requiring anticoagulation–saxophone study
Apixaban is an oral, direct, selective factor Xa (FXa) inhibitor1 and may be a treatment option for venous thromboembolism prevention in...
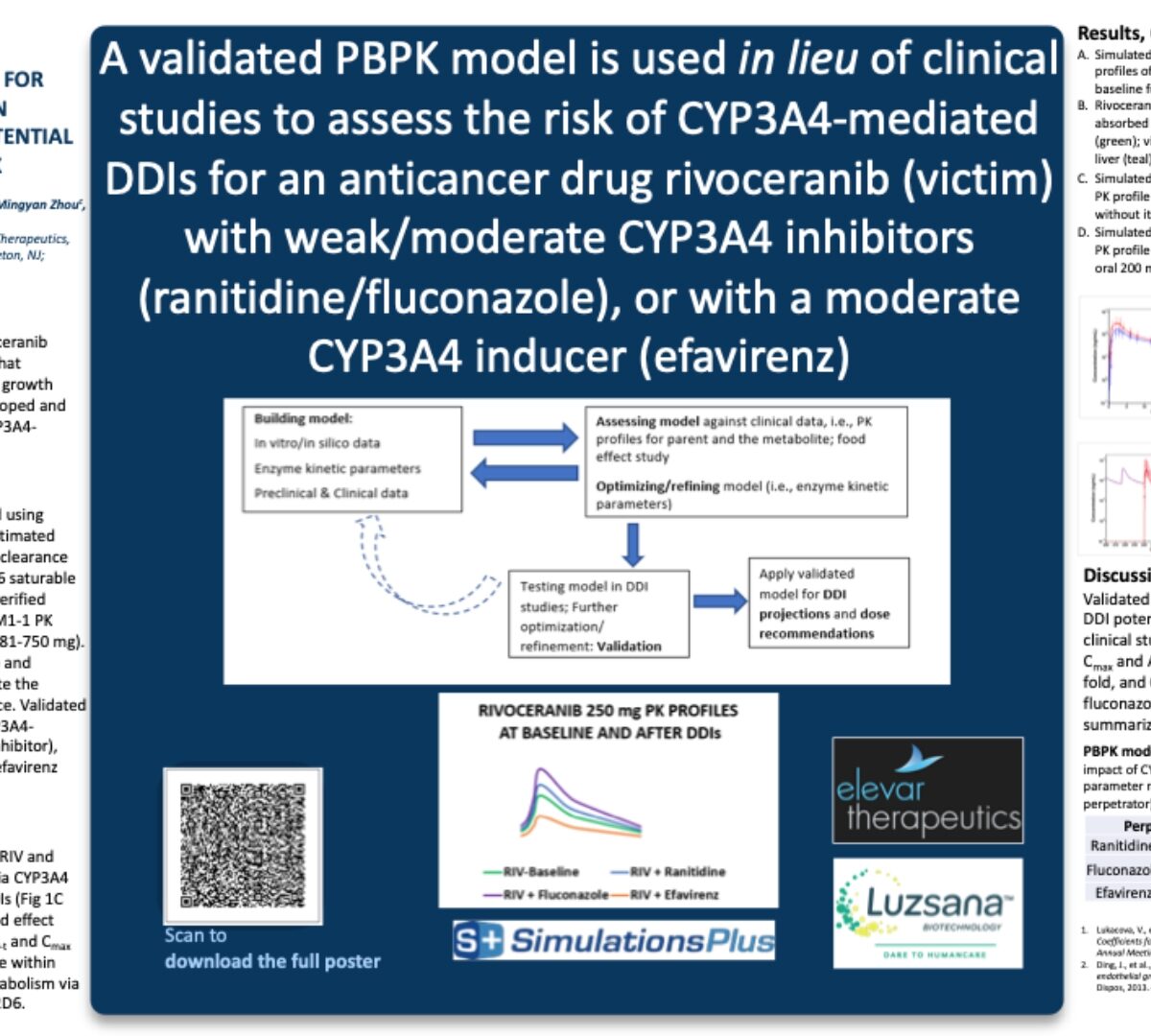
A validated PBPK model is used in lieu of clinical studies to assess the risk of CYP3A4-mediated DDIs for an anticancer drug rivoceranib (victim) with weak/moderate CYP3A4 inhibitors (ranitidine/fluconazole), or with a moderate CYP3A4 inducer (efavirenz)
A PBPK model for anticancer drug rivoceranib (RIV), a tyrosine kinase inhibitor (TKI) that selectively targets vascular endothelia growth factor...

Attenuations in adiponectin production over time restrict simulated efficacy for pegbelfermin in F3 NASH patients
Patients with non-alcoholic steatohepatitis (NASH) and fibrosis do not currently have options for...

Greater efficacy predicted for FGR1/beta-klotho receptor agonists that achieve 60% or greater increases in serum adiponectin
The FGFR1/beta-klotho receptor (FGFR1/KLB) in adipose has been demonstrated to be a...

Reduction of daily moderate alcohol intake predicted to decrease fibrosis stage in patients with non-alcholic steatohepatitis
Several recently completed clinical trials in NASH patients have included fibrosis stage reductions in...

Mathematical Representation of Drug-Induced Crystal Nephropathy Using a Quantitative Systems Toxicology Approach
Drugs may cause crystal nephropathy by precipitating within kidney tubules or inducing...

Mathematical Modeling of Renal Sodium, Potassium, and Glucose Dynamics in Diabetic and Non-Diabetic Simulated Populations
Type 2 diabetic (T2D) patients often exhibit reduced systolic and diastolic functions, which can place them at...