Clearing Up Myths About Aqueous Ionization of Drugs, Understanding the pH Dependence of Aqueous Solubility, & Understanding the pH Dependence of Partitioning

Case Study in Placebo Modeling and Its Effect on Drug Development
ADHD (attention-deficit/hyperactivity disorder) is a neurodevelopmental disorder characterized by symptoms of inattention, hyperactivity, and impulsivity associated with clinically significant impairment in functioning.

High-throughput prediction of fraction absorbed and bioavailability in silico
HTPK simulations run using purely in silico property estimates are in reasonable but imperfect agreement with experimental results in humans and rats.

In Silico-In Vitro Extrapolation for Dermal Exposure
Prediction of in vitro dermal delivery and drug in receptor fluid should be done using Nielsen model for vehicle evaporation...

Applying in silico-in vitro-in vivo extrapolation (IS-IVIVE) techniques to predict exposure and guide risk assessment
Combining QSAR, PBPK, and QST models is a powerful approach to getting more information out of your data investments early in product development.

Using Quantitative Systems Toxicology (QST) to Assess Drug Safety: The Experience of the DILIsimInitiative. ®
Drug-Induced Liver Injury (DILI) remains a major problem in drug development.
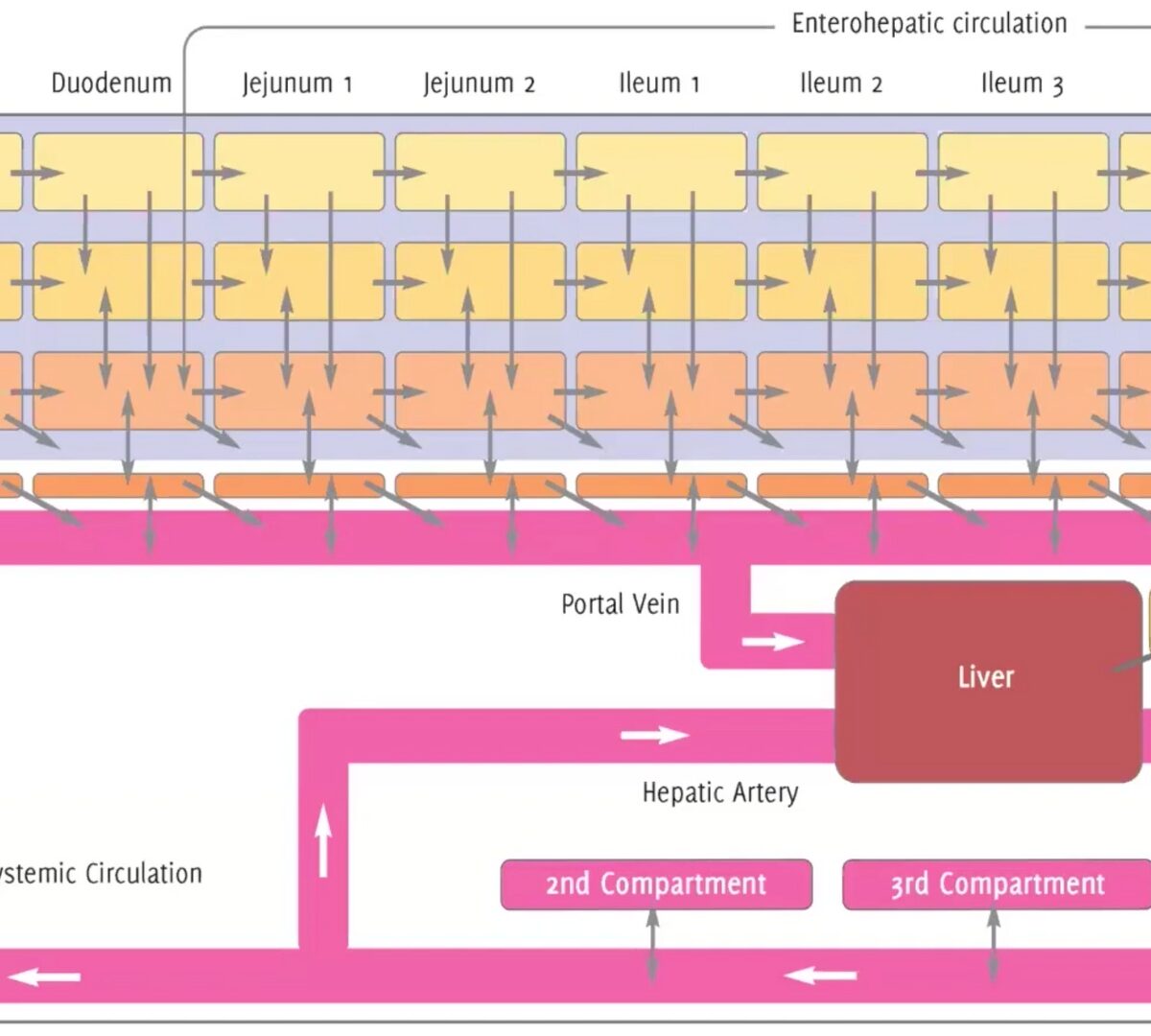
Applying Physiologically-Based Pharmacokinetic (PBPK) Modeling & Simulation to Assist with Pharmaceutical Research and Regulatory Submissions
Open communication between regulatory agencies, pharmaceutical companies, universities, and software providers will help identify new M&S applications.

Development of a Physiologically Based Pharmacokinetic Model for Raltegravir and Prediction of its Interactions with Rifampicin
Raltegravir (MK-0518; Isentress™), the HIV-1 integrase strand transfer inhibitor is approved for HIV-1 therapy in combination with other antiretroviral agents.
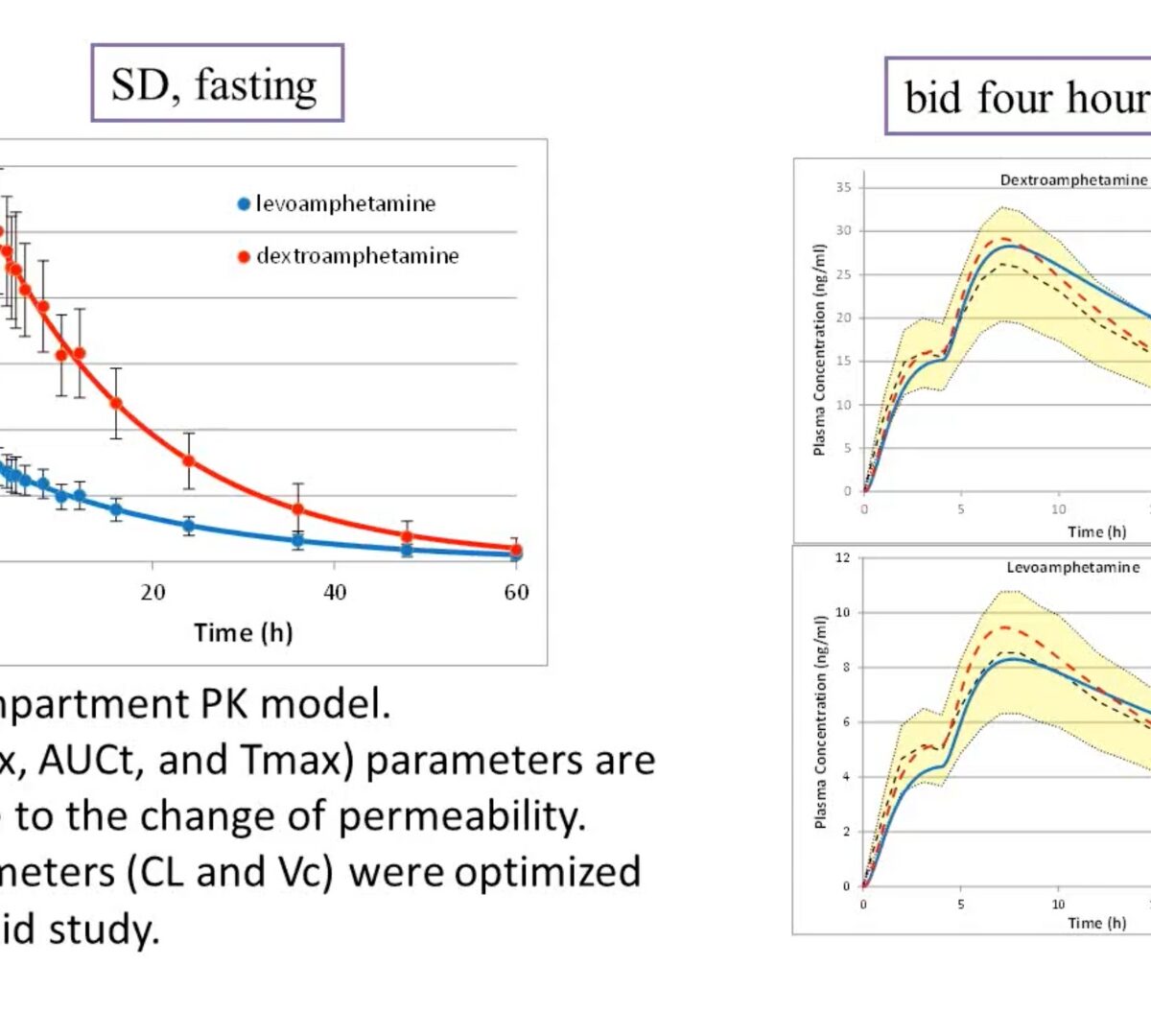
Application of PBPK Modeling in Generic Drug Evaluation
This GastroPlus™ User Group/FDA webinar provides a few examples of modeling and simulation in the OGD for the purpose of addressing biopharmaceutical performance questions for orally administered generic drug products. View slides from this webinar.


