DILIsym Services Is Using QSP and QST Modeling to Predict Efficacy and Safety of Drugs in Development
Development of Drugs to Treat NAFLD/NASH using Quantitative Systems Pharmacology Modeling
This session will provide scientific background and overview of the application of quantitative systems pharmacology (QSP) modeling in drug development to treat NALFD/NASH

Different Perspectives – Informing Drug Development Decision Making Through Complementary M&S Approaches
Although fundamental principles are different between PBPK and population PK approaches, both techniques have been used to study drug disposition during drug development.

Mechanistic PBPK modeling of special population groups – considerations and opportunities
Unwarranted studies, due to the general nature of regulatory guidelines, may be avoided.

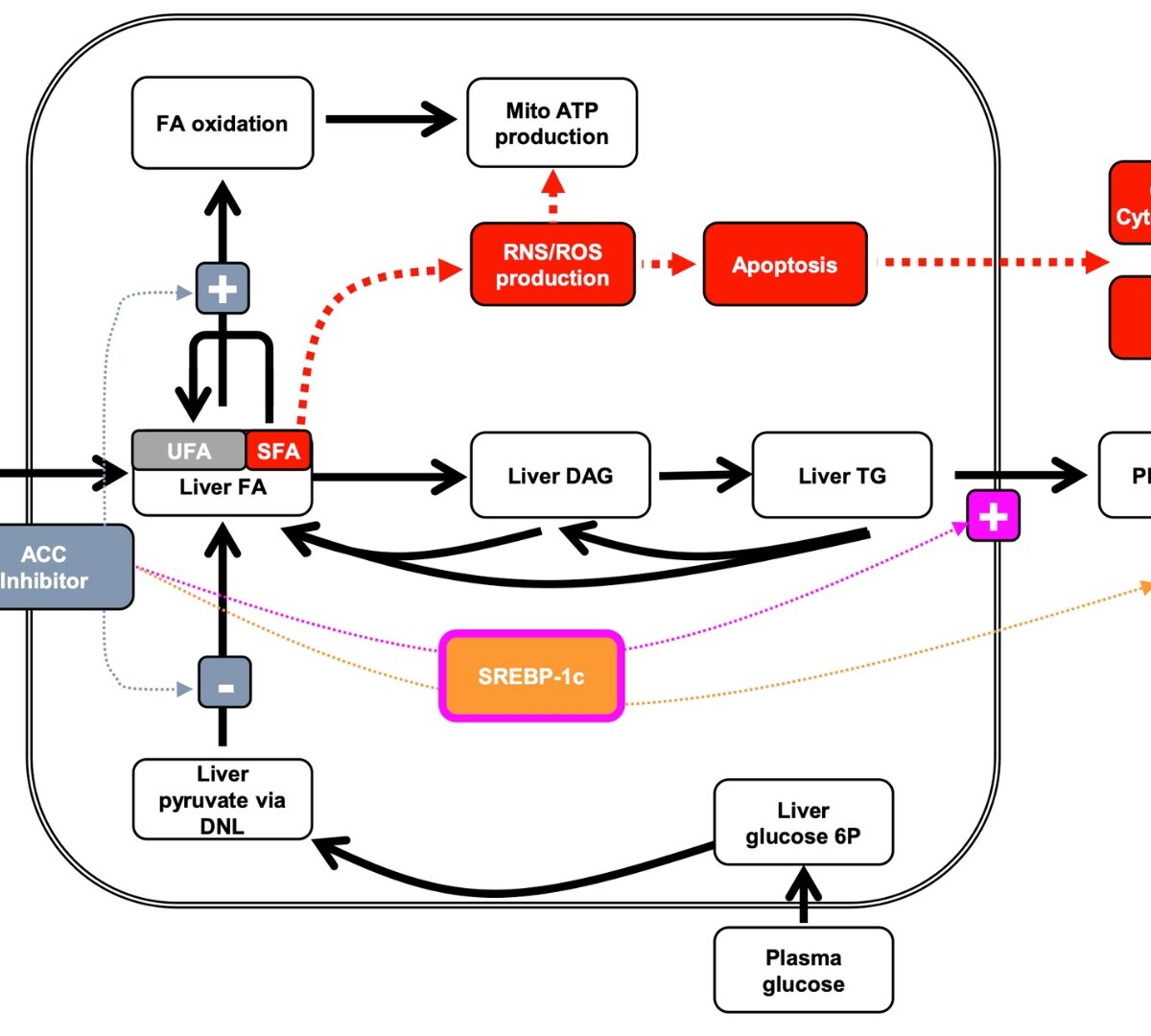
Utilizing DILIsym, a QST Platform, to Extract More from Your Data to Support Decisions
Recently Completed Clinical Trial Indicates that DILIsym Backup Compound Predictions Were Correct

New approach to regression uncertainty analysis and applications to drug design
When can you trust a decision/prediction from a machine learning model?

Using Quantitative Systems Toxicology (QST): Improving the Safety of Drugs While Reducing Animal Testing
QST modeling is having a significant impact on decision making in drug development.

How to obtain biowaivers for clinical trials using PBPK models, two case studies
FDA is open to proposals of using modeling approaches for bioequivalence (BE), or for new drugs,with the proper justification and model verification

Advancing current PBPK model applications to support internal development and regulatory decisions
Numbers of applications (NDA, ANDA) supported by PBPK modeling has increased significantly since 2008.

In Silico and in Vitro Simulations to Predict Idiosyncratic DILI: What is on the Horizon?
Multiple species: human, rat, mouse, and dog, Population variability, The three primary acinar zones of liver represented, Essential cellular processes represented to multiple scales in interacting submodels...

Assessing and Managing DILI Risk in Trials: A Consultant’s Perspective
DILI typically has drug-specific “signatures”: Hepatocellular, cholestatic, mixed (R-value), Latency “window,” Rate of progression, rate of resolution, Extra-hepatic manifestations (e.g. hypersensitivity signs)

How to Understand Aqueous Ionization and Its Influence on Key Physical Properties of Drugs
Part I “You Must Unlearn What You Have Learned”: Clearing Up Myths About Aqueous Ionization of Drugs

Validating property and metabolite predictions for some novel antimalarial compounds
Metabolite Assignment Considerations & Caveats: One compound can (and often does) give rise to two or more mass spectral (MS) peaks - but there should only be one HPLC peak per compound...

Developing PBPK for Ocular Delivery
Cooperation grant with the FDA (2014‐2019) a 4‐year funded collaborative project with the FDA Office of Generic Drugs on the development of mechanistic models for ocular delivery

Proprietary modeling platforms to support regulatory interactions: A vendor’s perspective
Proprietary modeling platforms are driving the vast majority of internal R&D activities at companies.

Assessing Effects of Sublingual BHV-0223 and Oral Riluzole on Liver Function Test Parameters
DILIsym is a mechanistic, mathematical model that has been constructed to support pharmaceutical risk assessment and decision making

Applying in silico-in vitro-in vivo Extrapolation (IS-IV-IVE) Techniques to Predict Exposure and Guide Risk Assessment
Applying IS-IV-IVE Techniques to Predict Exposure and Guide Risk Assessment

Game Changing: The Latest Developments in the Machine Learning/ PBPK/QST Modeling Space
Simulations Plus continues to lead in the areas of PBPK modeling to support regulatory submissions and alternatives to animal testing.

DILIsym 8A New Features for Predicting & Understand Drug-Induced Liver Injury
So how can DILIsym help my organization? Predict DILI liabilities beforehand and save $$$, Choose the lead candidate most likely to succeed from a DILI standpoint, Communicate with regulators on safety issues with information they have requested from others numerous times and from a platform they license (FDA) & keep patients safer.

