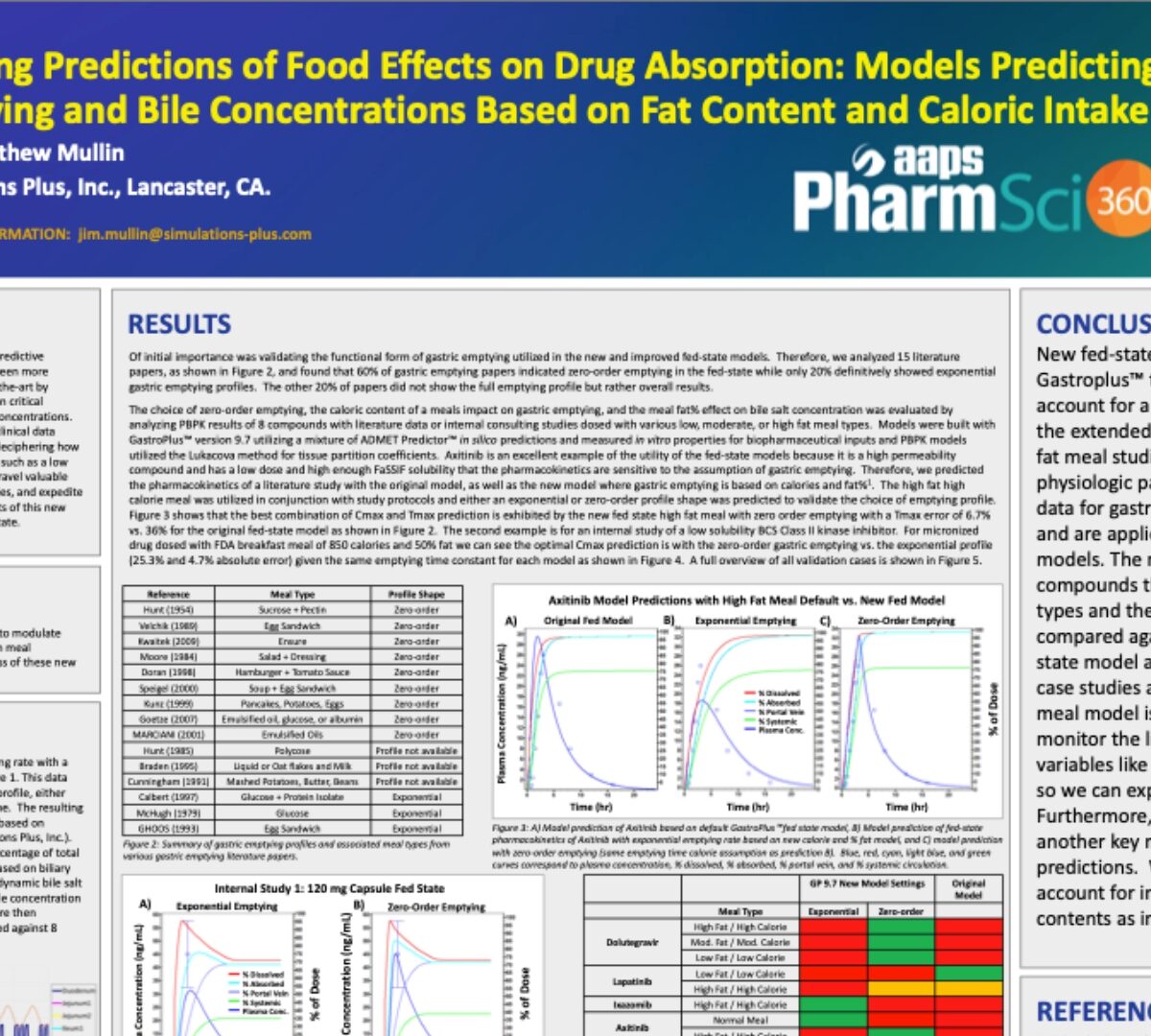
In the realm of pharmaceutical R&D, the quest for enhanced predictive accuracy and efficiency for Fed State PBPK models has never been more pressing.

Predicting absolute aqueous solubility by applying a machine learning model for an artificially liquid-state as proxy for the solid-state
In this study, we use machine learning algorithms with QM-derived COSMO-RS descriptors, along with Morgan fingerprints, to predict the absolute solubility of drug-like compounds.
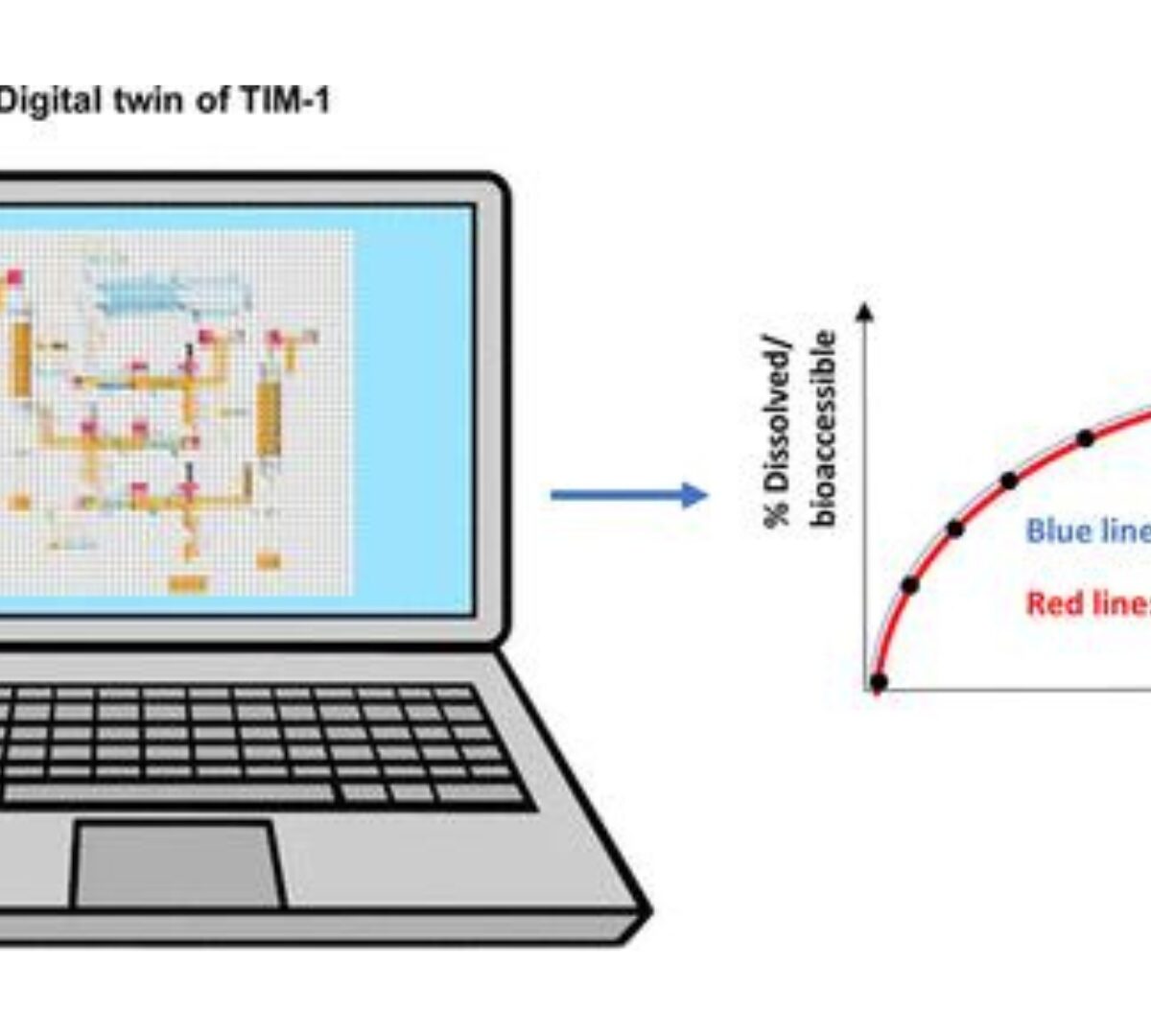
Digitalizing the TIM-1 Model Using Computational Approaches─Part Two: Digital TIM-1 Model in GastroPlus
A TIM-1 model is an in vitro gastrointestinal (GI) simulator considering crucial physiological parameters that will affect the in vivo drug release process.

Ionization Prediction Summit Webinar Series: Session 1 Fundamentals of Ionization
In the Ionization Prediction Summit webinar series, leading industry experts will explain ionization, how ionization modeling works, how ionization models are influenced by the large amounts of industrial data...
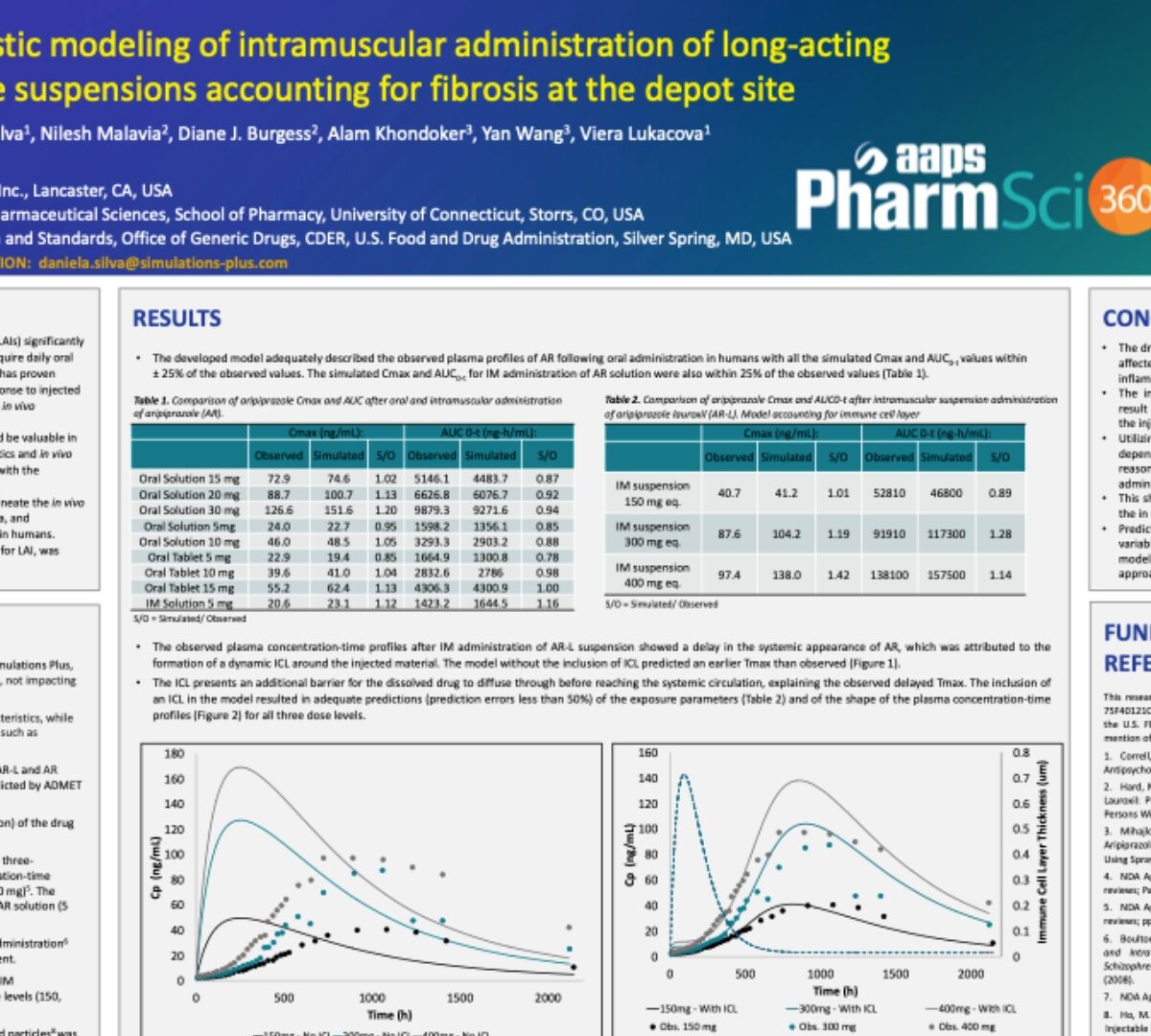
Mechanistic Modeling of Intramuscular Administration of Long-Acting Injectable Suspensions Accounting for Fibrosis at the Depot Site
Antipsychotic drugs formulated as long-acting injectables (LAIs) significantly improve patient compliance compared to regimens that require daily oral administration.
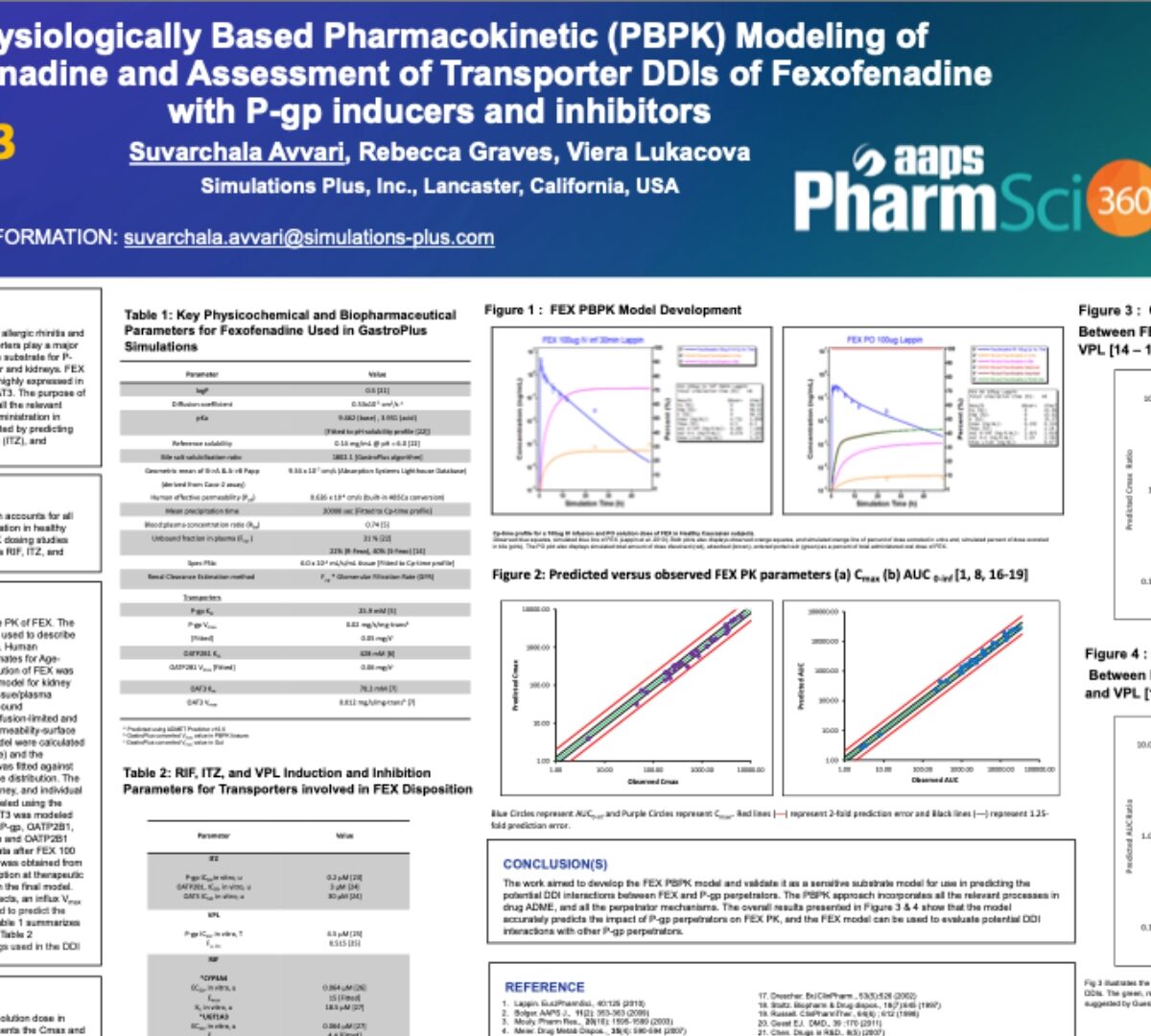
Physiologically Based Pharmacokinetic (PBPK) Modeling of Fexofenadine and Assessment of Transporter DDIs of Fexofenadine with P-gp inducers and inhibitors
Fexofenadine (FEX), a H1-receptor antagonist used in the treatment of allergic rhinitis and chronic idiopathic urticaria, undergoes minimal metabolism and transporters play a major role in its absorption and disposition.
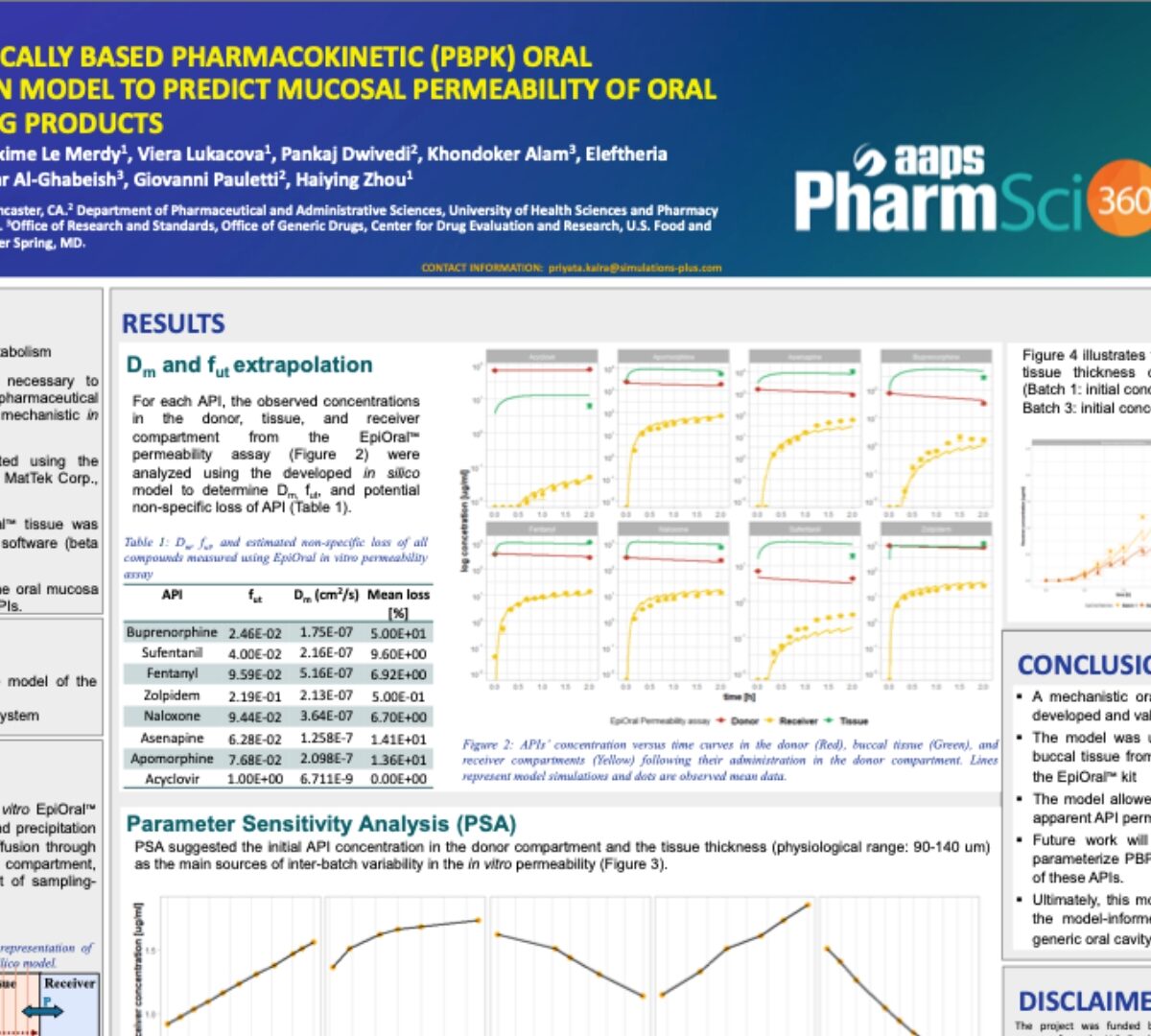
Physiologically Based Pharmacokinetic (Pbpk) Oral Absorption Model To Predict Mucosal Permeability of Oral Cavity Drug Products
Buccal delivery allows bypassing first-pass metabolism

Early Drug Discovery Timeline Acceleration
Unleash the Power of AI for Early Drug Discovery
ADMET Predictor Features & Functionality Comparison
Accurate and actionable ADMET predictions rely on premium data and proven algorithms.

AIDD Pythia Flyer
Use CDIAM and the AIDD platform in ADMET Predictor to take your program from target indication to optimized lead candidate
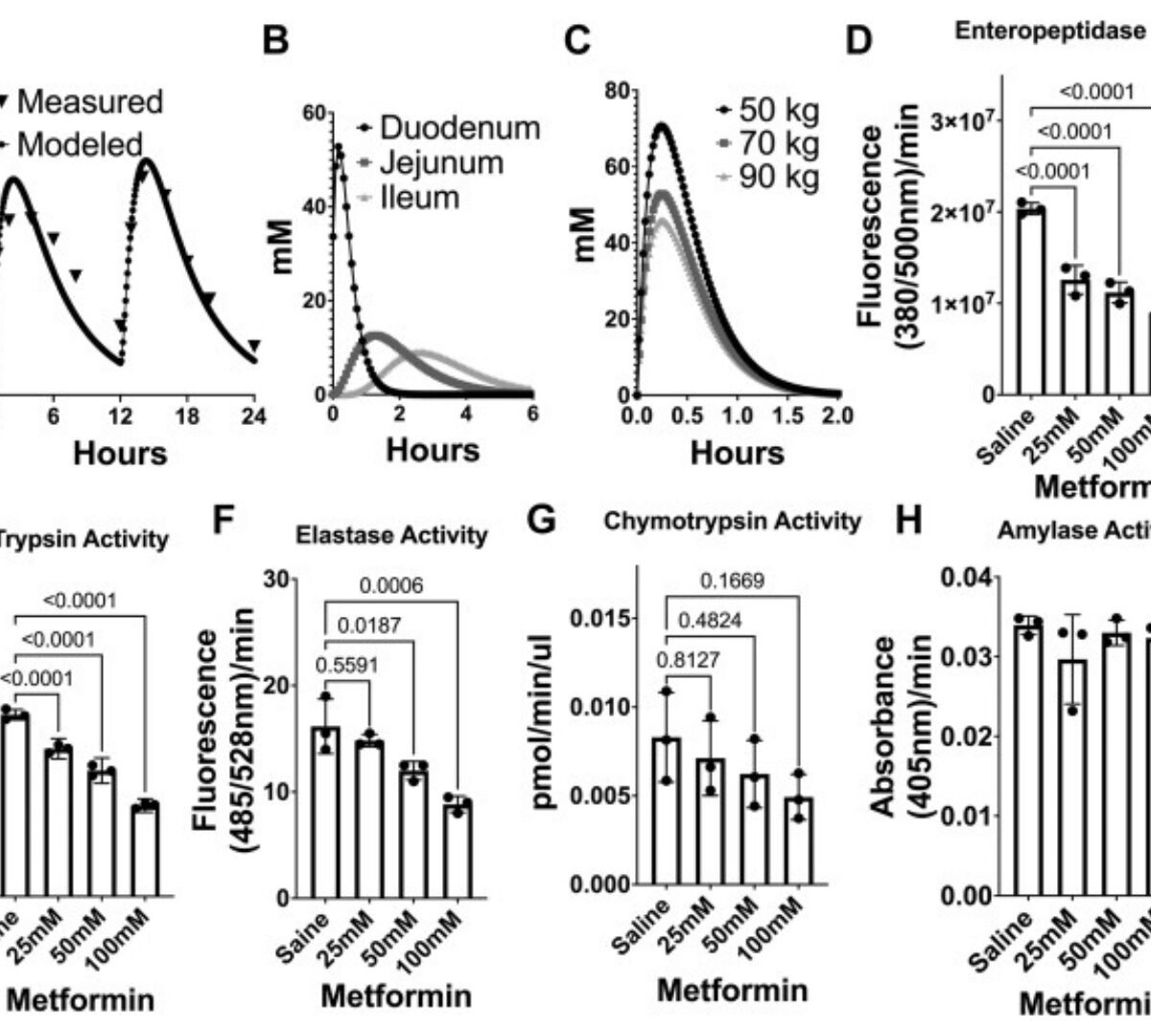
Metformin inhibits digestive proteases and impairs protein digestion in mice
Metformin is among the most prescribed medications worldwide and the first-line therapy for type 2 diabetes.
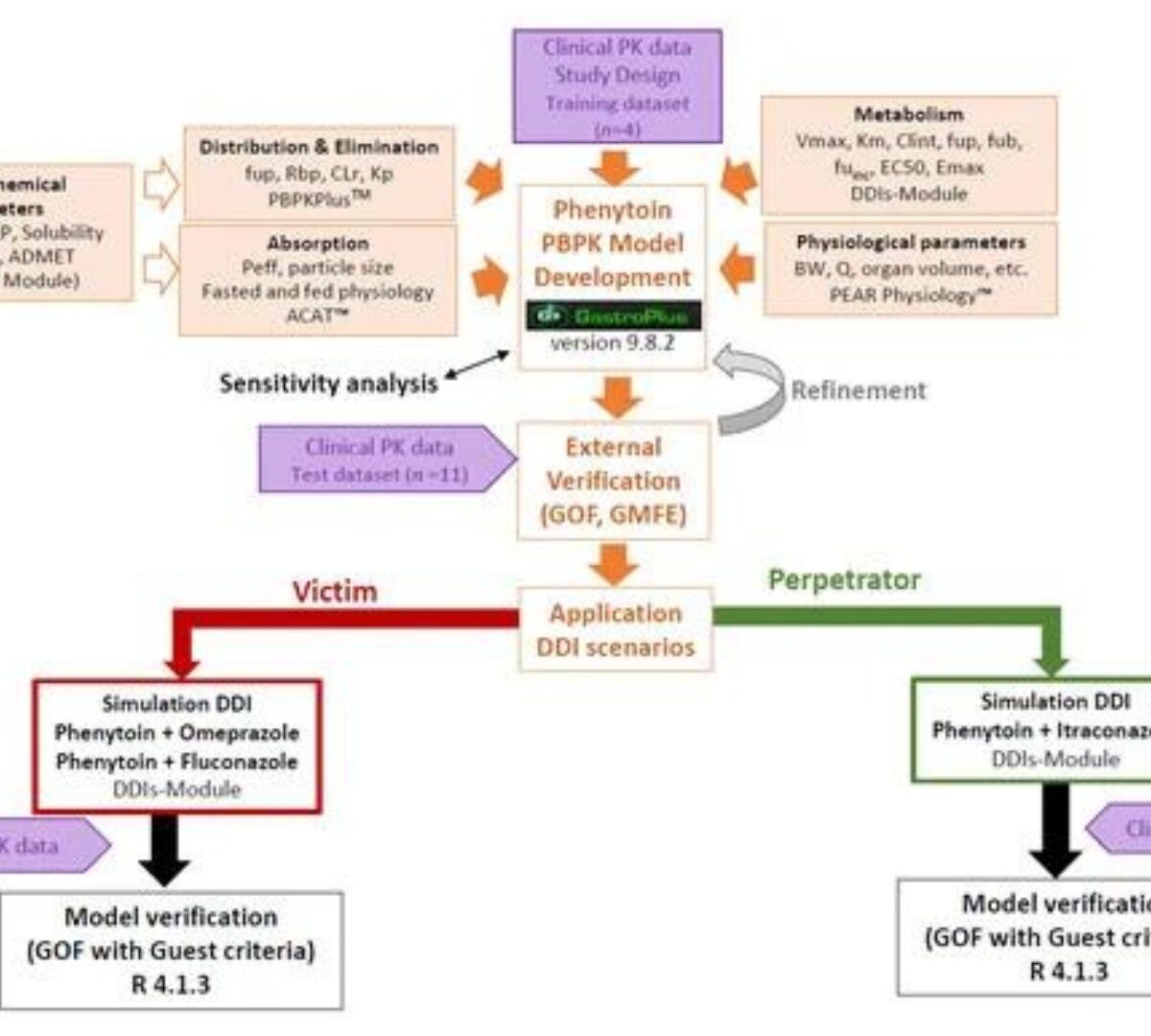
Comprehensive Physiologically Based Pharmacokinetic Model to Assess Drug–Drug Interactions of Phenytoin
Regulatory agencies worldwide expect that clinical pharmacokinetic drug–drug interactions (DDIs) between an investigational new drug...

October 2023 News/Events
Using ionization modeling to inform drug discovery and decision-making

Capture and Use of Meaningful Data for Population PK/PD Analysis with Non-Linear Mixed Effects Modeling
Your model is only as good as your data!

October 2023 GastroPlus Newsletter
GastroPlus® Newsletter October 2023
NAFLDsym® Flyer
Predict and prevent metabolic dysfunction-associated fatty liver disease and MASH.
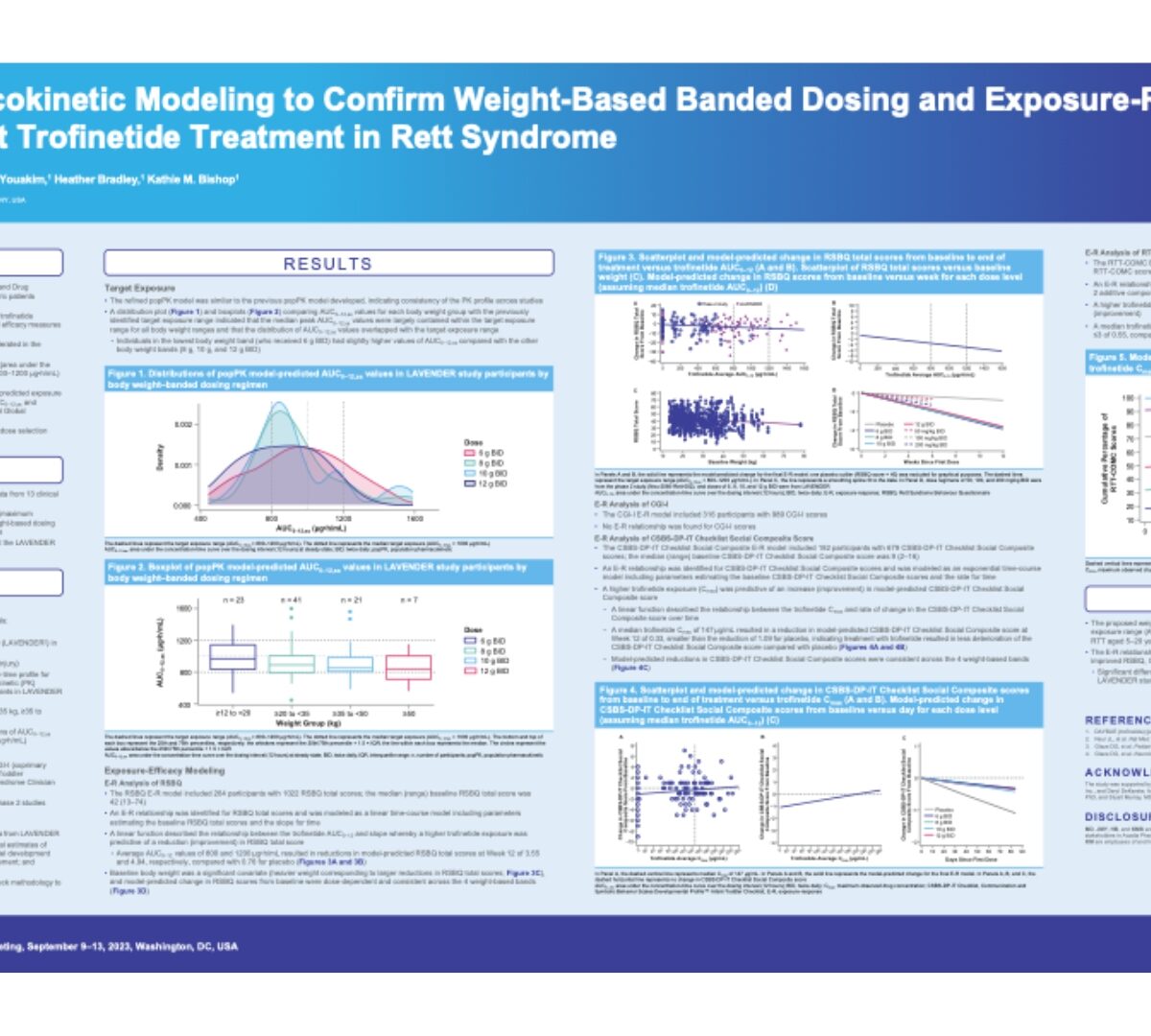
Population pharmacokinetic modelling to confirm weight-based banded dosing and exposure-response efficacy analyses to support trofinetide treatment in rett syndrome
Trofinetide, a synthetic analog of glycine-proline-glutamate, was approved by the US Food and Drug Administration in March 2023 for the treatment of...
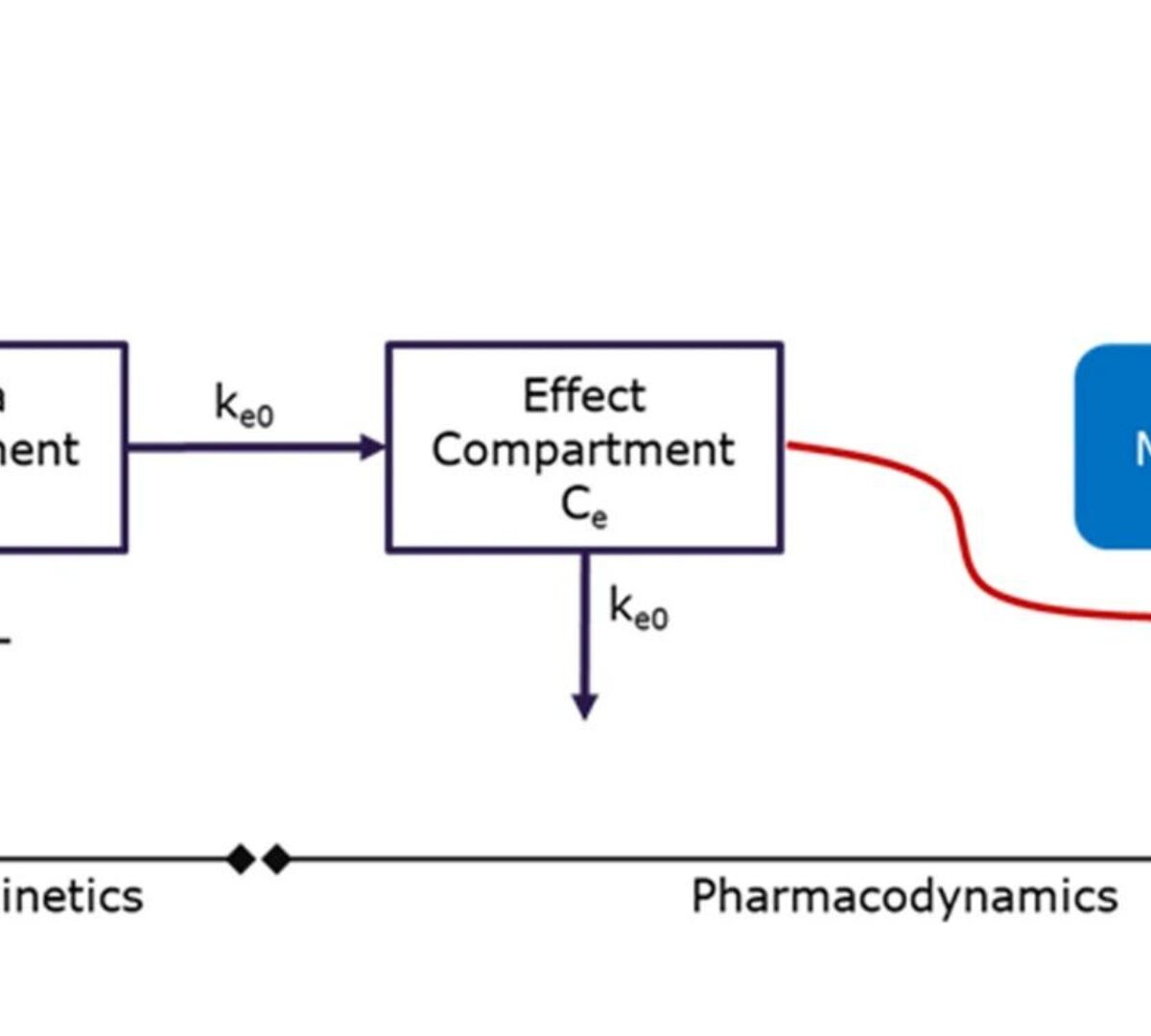
Preclinical Pharmacokinetics and Translational Pharmacokinetic/Pharmacodynamic Modeling of M8891, a Potent and Reversible Inhibitor of Methionine Aminopeptidase 2
M8891 is a selective and reversible inhibitor of methionine aminopeptidase 2 (MetAP2).

Pharmacokinetics of the novel 5-HT4 receptor agonist, DA-6886, in dogs
The pharmacokinetics of a new 5-hydroxytryptamine receptor 4 agonist, DA-6886, intended for the treatment of constipation-predominant irritable bowel syndrome...
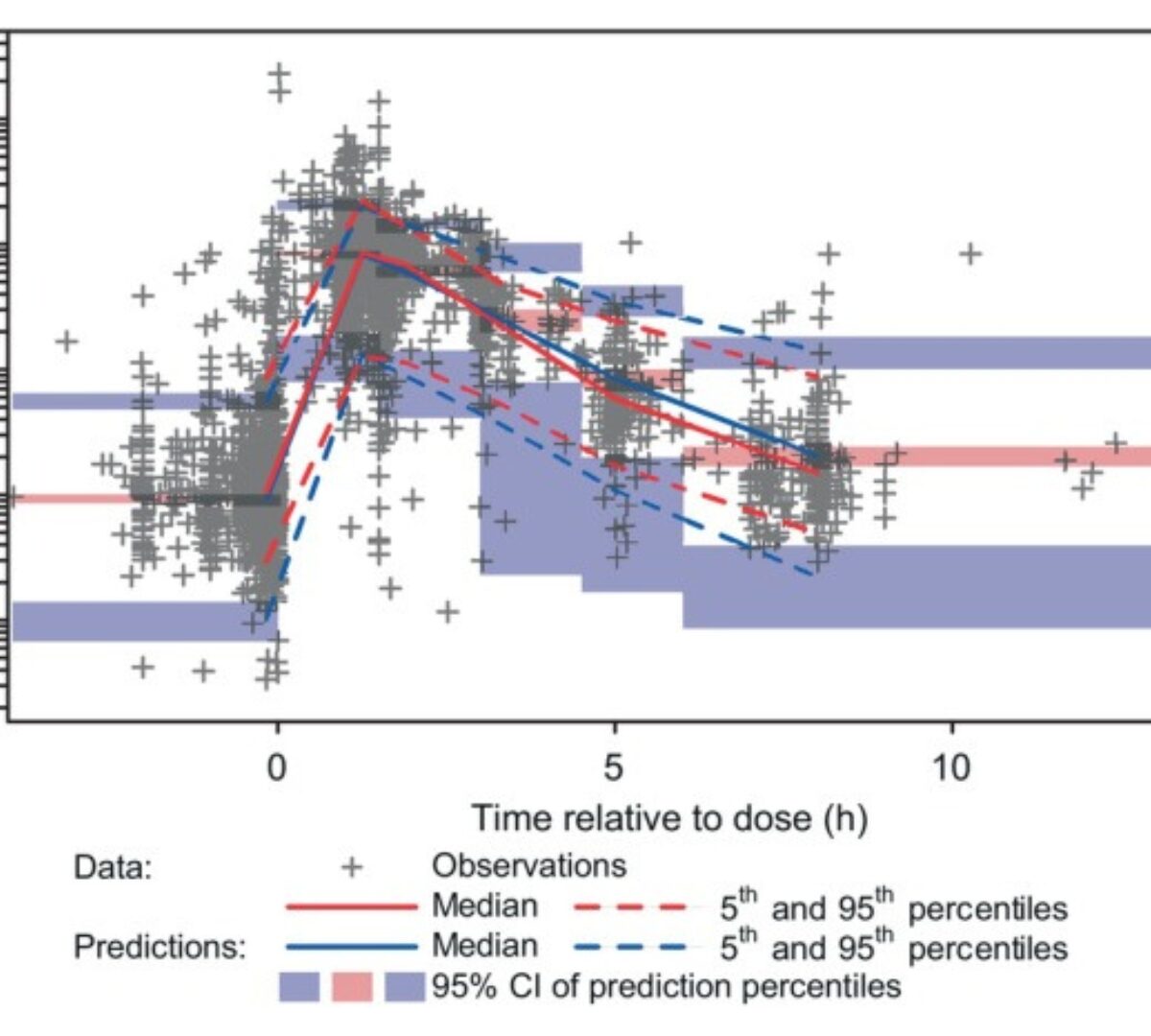
Population Pharmacokinetics of Molnupiravir in Adults With COVID-19: Lack of Clinically Important Exposure Variation Across Individuals
Effective antiviral treatments for coronavirus disease 2019 (COVID-19) are needed to reduce the morbidity and mortality associated with severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) infection, particularly...