The prominence of biologic drugs has rapidly gained traction and has delivered life-changing...
Lunch & Learn – SOT 63rd Annual Meeting and ToxExpo
SOT 2024: What's New at Simulations Plus, Your Partner in Winning, for Exposure and Safety Assessment

Investigating the Potential Hepatotoxicity of ORM-48824 in a Quantitative Systems Toxicology Platform for Liver Safety, DILIsym®
ORM-48824 is a transient receptor potential Ankyrin-1 (TRPA1) antagonist and was initially being developed for patients with diabetic neuropathic pain...

Prediction of Multidrug Resistance Protein 3 (MDR3) Inhibition-mediated Cholestatic Drug-induced Liver Injury (DILI) Using Quantitative Systems Toxicology (QST) Modeling
DILI is a primary cause of acute liver failure and reason for the termination of drug development programs...

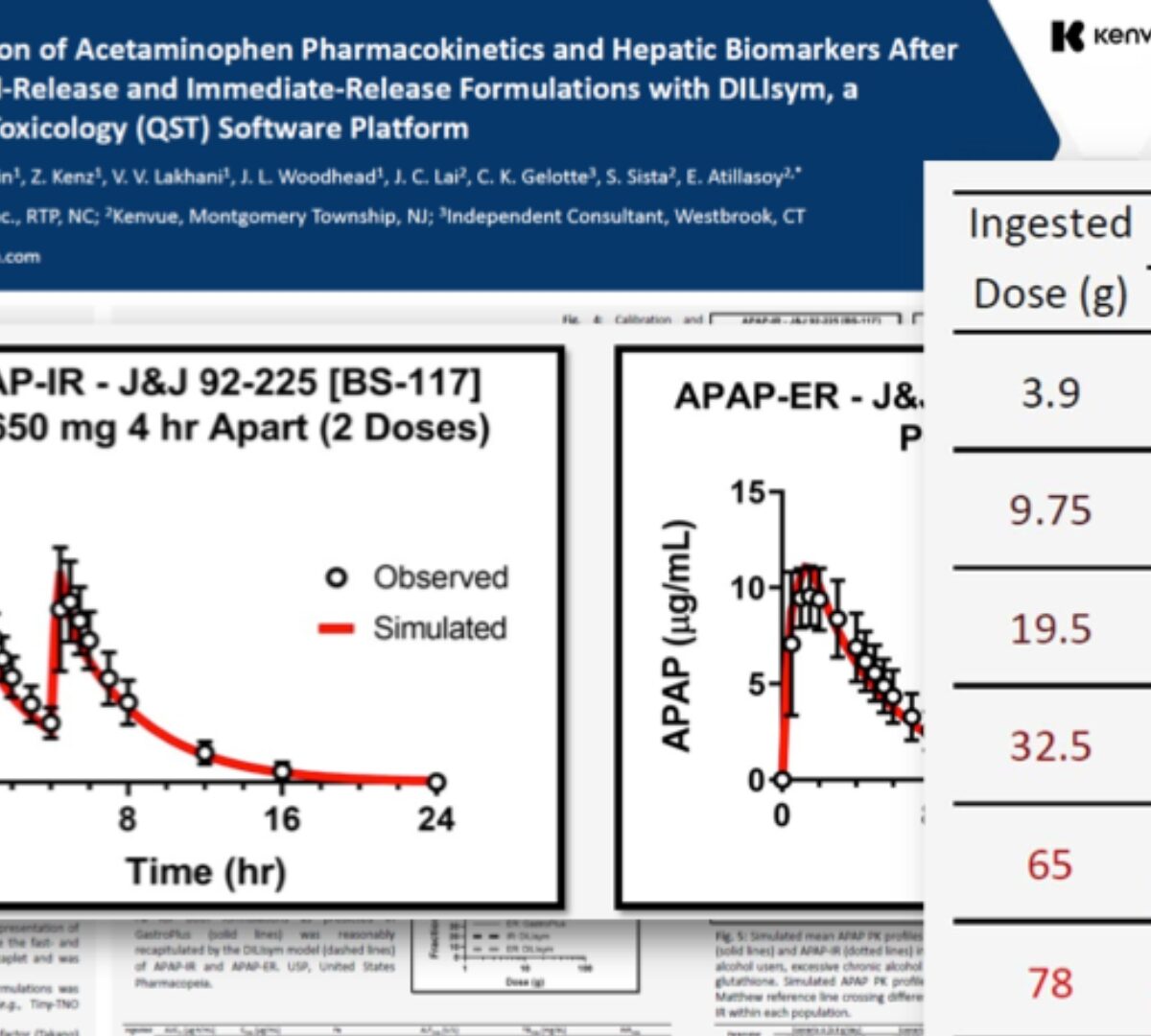
Modeling and Simulation of Acetaminophen Pharmacokinetics and Hepatic Biomarkers After Overdoses of Extended-Release and Immediate-Release Formulations with DILIsym, a Quantitative Systems Toxicology (QST) Software Platform
The analgesic/antipyretic acetaminophen (APAP) has multiple formulations including immediate-, modified-, and extended-release preparations...
OBESITYsym Flyer
Lose the Nausea in Your Weight Loss Therapies

Drug-like Properties of Serial Phenanthroindolizidine Alkaloid Compounds: ADMET Characteristic Prediction and Validation
Phenanthroindolizidine alkaloids (PAs) are a series of compounds that have been isolated from traditional herbal medicines and have significant therapeutic potential, such as anti-arthritic, anti-viral, anti-inflammatory, and anti-glioma effects in vitro and in vivo.

Simulations Plus to Present at the KeyBanc Capital Markets Virtual Life Sciences & MedTech Investor Forum
Simulations Plus to Present at the KeyBanc Capital Markets Virtual Life Sciences & MedTech Investor Forum

March 2024 News/Events
International Perspectives on Model-Informed Precision Dosing

March 2024 GastroPlus Newsletter
GastroPlus® Monthly Newsletter 2024

A Novel Stability-Indicating RP-HPLC Method for the Simultaneous Estimation and In Vitro and In vivo Evaluation: Curcumin and Naringin Co-amorphous System
Curcumin (CUR) is a phytochemical widely used in food industries, cosmetics, and in the treatment of various ailments. It is a polyphenol derived from turmeric and is often considered the golden spice.

Screening inhibitors against the Ef-Tu of Fusobacterium nucleatum: a docking, ADMET and PBPK assessment study
The oral pathogen Fusobacterium nucleatum has recently been associated with an elevated risk of colorectal cancer (CRC), endometrial metastasis, chemoresistance, inflammation, metastasis, and DNA damage, along with several other diseases.

Women in Science: Dr. Qi (Joy) Yang, Assistant Director of Pharmacometrics
According to an article published by United Nations News last year, women in STEM are still not the norm. Only one in three researchers worldwide is female.

Physiologically Based Pharmacokinetic Modeling for Confirming the Role of CYP3A1/2 and P-glycoprotein in Detoxification Mechanism Between Glycyrrhizic Acid and aconitine in Rats
Fuzi, an effective common herb, is often combined with Gancao to treat disease in clinical practice with enhancing its efficacy and alleviating its toxicity.

Prediction of Human Pharmacokinetics of E0703, a Novel Radioprotective Agent, Using Physiologically Based Pharmacokinetic Modeling and an Interspecies Extrapolation Approach
E0703, a new steroidal compound optimized from estradiol, significantly increased cell proliferation and the survival rate of KM mice and beagles after ionizing radiation.

Weight Loss Without Nausea: How QSP Modeling Can Optimize Obesity Treatments
Ozempic and Wegovy have been in the news for months as the first highly effective weight loss drugs on the market. However, a noted side effect—nausea—can make it difficult for patients to adhere to proper use.

Simulations Plus and the University of Bath Awarded New FDA Grant
Partnership will produce an enhanced, validated dermal PBBM/PBPK model to inform product development and bioequivalence decisions.

Discovery of TRPA1 Antagonist GDC-6599: Derisking Preclinical Toxicity and Aldehyde Oxidase Metabolism with a Potential First-in-Class Therapy for Respiratory Disease
Transient receptor potential ankyrin 1 (TRPA1) is a nonselective calcium ion channel highly expressed in the primary sensory neurons, functioning as a polymodal sensor for exogenous and endogenous stimuli, and has been implicated in neuropathic pain and respiratory disease.

Regulatory Requirements and Applications of Physiologically Based Pharmacokinetic Models
Physiologically based pharmacokinetic (PBPK) models have become a key tool to reduce the uncertainty and assure the best benefit-risk decisions in a model-informed drug discovery and development process.
