
GastroPlus® Additional Dosage Routes Brochure
Trust Our Gut... and Model Everywhere!
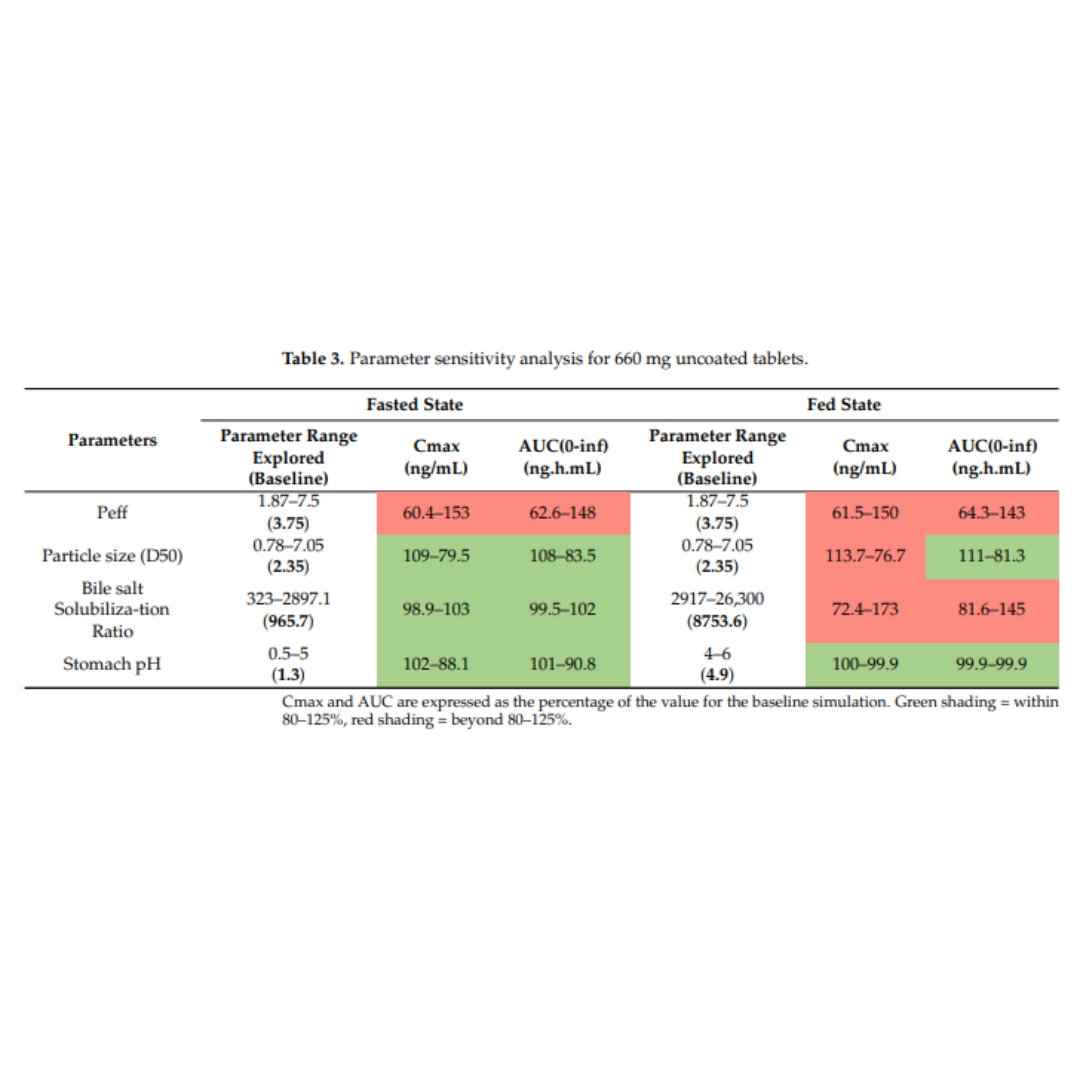
Physiologically Based Biopharmaceutics Modeling of Food Effect for Basmisanil: A Retrospective Case Study of the Utility for Formulation Bridging
Basmisanil, is a lipophilic drug substance, exhibiting poor solubility and good permeability (BCS class 2). A validated physiologically based biopharmaceutics model (PBBM)...

Review and Chemoinformatic Analysis of Ferroptosis Modulators with a Focus on Natural Plant Products
Ferroptosis is a regular cell death pathway that has been proposed as a suitable therapeutic target in cancer and neurodegenerative diseases.

Oral administration of VDAC1-derived small molecule peptides increases circulating testosterone levels in male rats
Cholesterol is the precursor of all steroid hormones, and the entry of cholesterol into the mitochondria is the rate-limiting step of steroidogenesis.

Simulations Plus Reports First Quarter Fiscal 2023 Financial Results
Total revenue of $12 million; Diluted Earnings Per Share (EPS) of $0.06; Both in line with fiscal 2023 guidance and Q1 expectations for revenue seasonality shift.
Provides capital allocation strategy update focusing on corporate development activities, $50 million share repurchase program, and internal investment

Regulatory utility of physiologically based pharmacokinetic modeling for assessing food impact in bioequivalence studies: A workshop summary report
This workshop report summarizes the presentations and panel discussion related to the use of physiologically based pharmacokinetic (PBPK) modeling approaches for food...

Structure-activity relationship read-across and transcriptomics for branched carboxylic acids
The purpose of this study was to use chemical similarity evaluations, transcriptional profiling, in vitro toxicokinetic data, and physiologically based pharmacokinetic (PBPK)...

In-Depth Analysis of Physiologically Based Pharmacokinetic (PBPK) Modeling Utilization in Different Application Fields Using Text Mining Tools
In the past decade, only a small number of papers have elaborated on the application of physiologically based pharmacokinetic (PBPK) modeling across different areas.

Best Company for Women Award
Simulations Plus is excited to announce that we are the recipients of a Comparably Award for “Best Company for Women” in 2022.

Pharmacokinetic Basis for Using Saliva Matrine Concentrations as a Clinical Compliance Monitoring in Antitumor B Chemoprevention Trials in Humans
This study reports the first clinical evidence of significantly high secretion of matrine in a multi-component botanical (Antitumor B, ATB) into human saliva from the systemic...
