Fluconazole is an antifungal agent widely used in the clinical setting for the treatment of candidiasis and meningitis. It undergoes minimal metabolism and is excreted renally(1). Fluconazole is a moderate…

Modeling Drug Disposition in Ocular Tissues following Topical Eye Drops and Intravitreal Injection
The purpose of this study was to model the ocular absorption, distribution and clearance of clonidine and voriconazole from topical and intravitreal applications, respectively. Clonidine is a potent…

Development of a Steady-State Exposure-Response Model for Exenatide Once Weekly
Exenatide is dosed as a subcutaneous (SC) injection of 5 and 10 μg twice daily (BID) before main meals and is indicated for the treatment of type 2 diabetes mellitus in patients failing to achieve adequate…

Omeprazole: Physiologically Based Pharmacokinetic (PBPK) Modeling and Prediction of Drug-Drug Interactions (DDI)
To optimize a PBPK model of omeprazole for prediction of DDIs with respect to polymorphic expression of CYP enzymes. Omeprazole absorption and pharmacokinetics were simulated using GastroPlus™.

Azole Antifungals: Physiologically-Based Pharmacokinetic (PBPK) Modeling and Prediction of Drug-Drug Interactions (DDIs)
Develop PBPK models for azole antifungals for prediction of DDIs. The absorption and pharmacokinetics of azole antifungals were simulated using GastroPlus™. The program's Advanced Compartmental and…

Azole Antifungals: Physiologically-Based Pharmacokinetic (PBPK) Modeling and Prediction of Drug-drug Interactions (DDIs)
Download the poster presented at the Rosenon conference in 2009 on the development of PBPK models for common azole antifungals and DDI predictions

Prediction of drug-drug interaction (DDI) between cilostazol and substrates or inhibitors of CYP 2C19 and 3A4
The aim of this study was to validate the utility of physiologically based pharamcokinetic (PBPK) models fore predictioin of DDI between cilostazol, kectoconazole, omeprazole and quindine.
General Approach to Calculation of Tissue:Plasma Partition Coefficients for Physiologically Based Pharmacokinetic (PBPK) Modeling
To conduct a comprehensive evaluation of methods for calculation of tissue/plasma partition coefficients with a focus on correct prediction of volume of distribution and recommendation for a general…

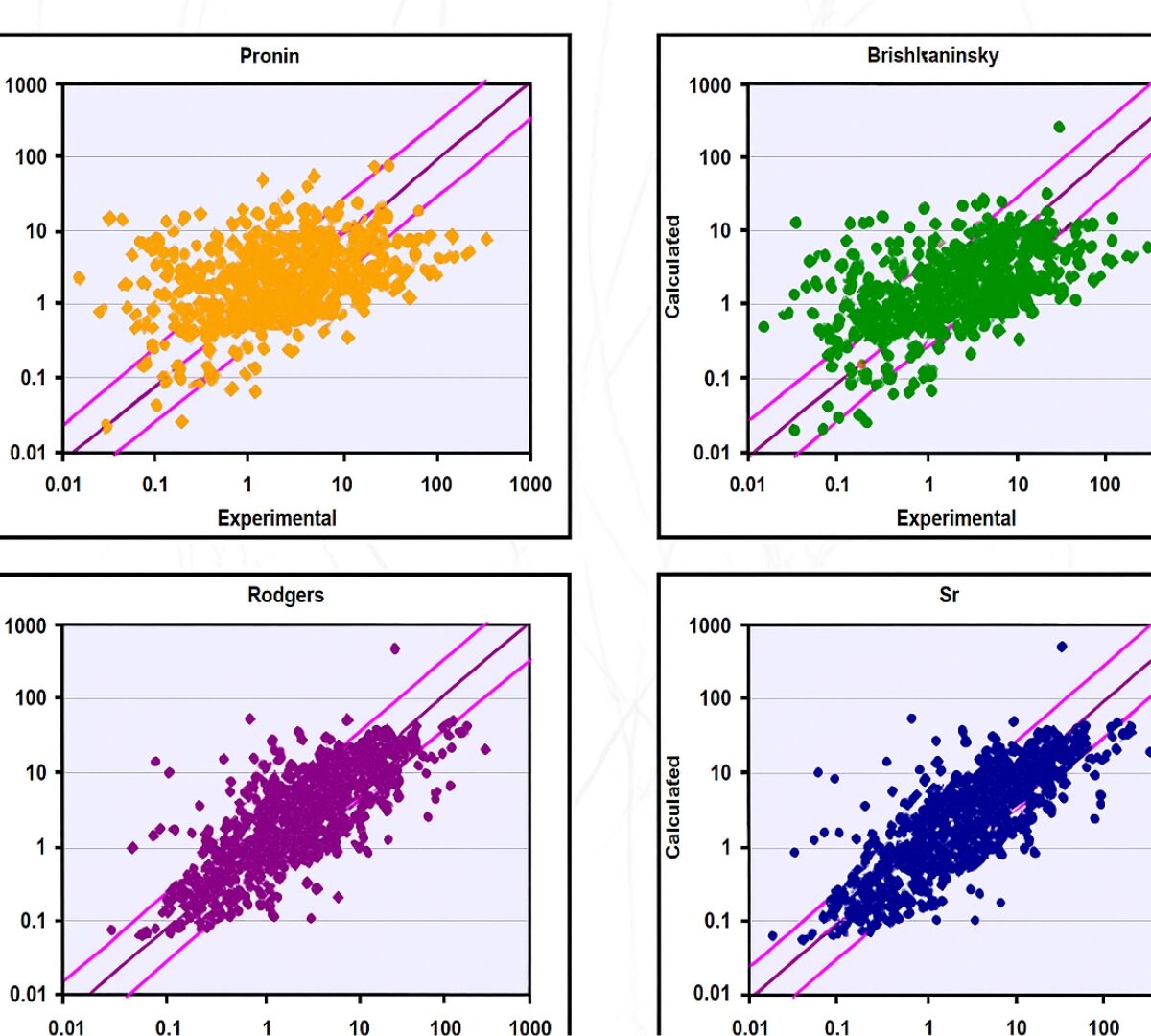
Role of Fraction Unbound in Plasma in Calculations of Tissue:Plasma Partition Coefficients
Previous investigations have shown that the Rodgers and Rowland method [Rodgers 2007] for prediction of tissue:plasma partition coefficients (Kps) provides good prediction for compounds with low to moderate…

Level A IVIVC Using a Comprehensive Absorption/PBPK Model for Metoprolol
Wagner-Nelson, Loo-Riegelman, numerical deconvolution, and convolution-based methods are conventional ways to form an in vitro-in vivo correlation (IVIVC). The ultimate goal for forming an IVIVC is to…

Modeling Effects of Exenatide on the Pharmacokinetics of Acetaminophen, Digoxin, and Warfarin
Exenatide, a 39-amino acid peptide used for treatment of type 2 diabetes, is known to inhibit gastric emptying and as a result to alter the absorption of orally administered concomitant medications.

Mechanistic Modeling of Metoprolol Absorption and Pharmacokinetics from Immediate and Modified Release Formulations
As one of the most widely used b-blocking agents, metoprolol is also a popular drug in research studies. A number of published studies describe the pharmacokinetics as well as the pharmacodynamics of…

PBPK Modeling of Metoprolol and Its Metabolites
Develop a model describing absorption and pharmacokinetics of metoprolol and the formation and pharmacokinetics of its metabolites.

Simulation of Sublingual and Gastrointestinal Absorption of Nifedipine
To develop an integrated model for drug absorption from the oral cavity. The model simulates combined absorption of a drug from both the oral cavity and the gastrointestinal tract.

Simulation of Gabapentin Absorption and Bioavailability in Pediatric Patients
To fit an absorption-pharmacokinetic model for simulation of Gabapentin in adult and pediatric populations. The model will be able to describe the nonlinear dose dependence of absorption mediated by an amino…

Population Pharmacokinetics of Bendamustine and Metabolites in Patients With Indolent Non-Hodgkin Lymphoma
Bendamustine is a bifunctional alkylating agent in development as monotherapy and in combination with other agents in the treatment of indolent non-Hodgkin lymphoma (NHL).

Model Feasibility Assessment as a Driver for Model-Based Drug Development
Complex pharmacometric analyses raise concerns about cost, time, and reliability of the modelbuilding process (MBP). The goal was to use a model feasibility assessment (MFA) process to improve the performance…

PK/PD Modeling of Adinazolam
Develop a model for prediction of the pharmacokinetic (PK) and pharmacodynamic (PD) profiles of Adinazolam from IV, immediate release oral, and controlled release oral formulations.

Influence Of Estimation Of Inter-Occasion Variability On Detection Of Time-Varying Covariates
To explore the influence of the estimation of inter-occasion variability (IOV) on the ability to detect time-varying covariates influencing PK parameters from a population PK (PPK) analysis using NONMEM.

Plasma Pharmacokinetic (PK) Profile of Two Consecutive Doses of Ferumoxytol in Healthy Subjects
Ferumoxytol is a carbohydrate-coated superparamagnetic iron oxide nanoparticle being evaluated for safety and efficacy in two distinct areas of medical need.
