One of the key areas for optimization in the drug development process is the discovery stage. Traditional methods are time-consuming and often produce leads that ultimately fail.

Application of PBBM and virtual bioequivalence to support formulation development and define dissolution specification, two case studies
During this webinar, we will shed light on the key benefits and practical implications of utilizing in-silico modeling for formulation development, biopharmaceutical predictions, and bioequivalence studies.

QSP: The Best Kept Secret for Increasing the Technical Probability of Success in Clinical Trials & Enhancing Regulatory Submissions
Quantitative systems pharmacology (QSP) is no longer an “emerging field.”

Better Together: AI/ML, PBPK and QSP/QST Modeling in Drug Discovery & Development
Modeling and simulation offer useful predictions that can help guide your drug development program—but what if you could get even more out of the available technology?

In silico modeling as a support tool in the development of new products in the generic pharmaceutical industry: case studies of the application of DDDPlus™ and GastroPlus® software
During this webinar, we will shed light on the key benefits and practical implications of utilizing in silico modeling for formulation optimization, biopharmaceutical predictions, and bioequivalence studies

ADMET Predictor® 11 is Here: AI & ML for Drug Discovery and Development
Your competitors are using AI and machine learning for ADMET prediction—are you?
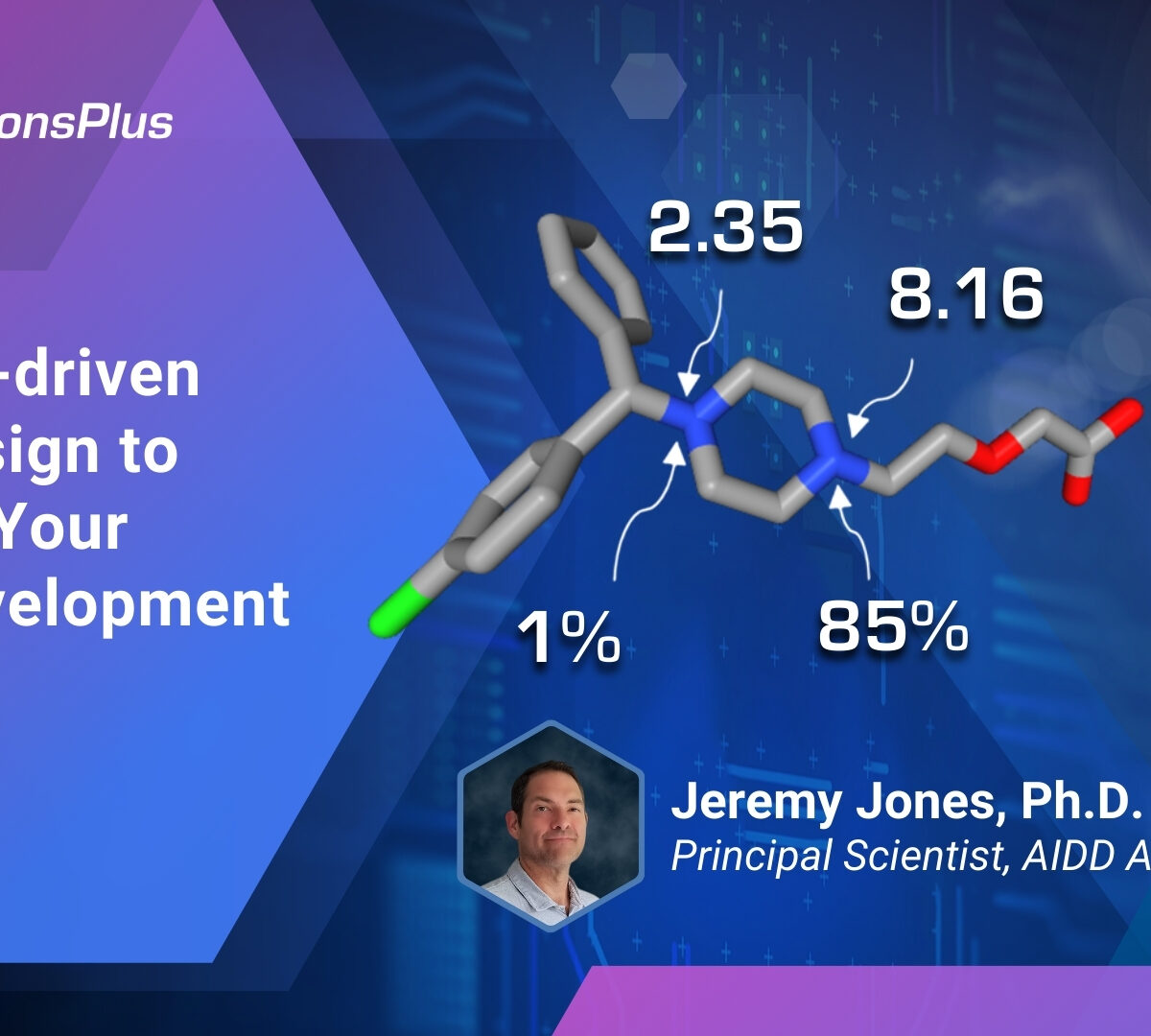
Using AI-driven Drug Design to Shorten Your Drug Development Process
In this webinar, Dr. Jeremy Jones, Principal Scientist, will discuss how artificial intelligence (AI) can be used in the drug discovery and development process to identify viable candidate molecules and shorten time to market.
Using GastroPlus®, PBBM and PKPD to Define Dissolution Safe Space in Support of Registration Specifications
If you’re curious how GastroPlus could be used to define dissolution safe space for your own projects, stream now.

PBPK Modeling for Identifying and Mitigating Absorption Risks in Early Drug Development
Physiologically based pharmacokinetic (PBPK) models represent animals and humans virtually as a collection of organs and tissues, each defined by a system of mathematical equations
What’s New in MonolixSuite 2023
Discover the new functionalities of MonolixSuite 2023 in this webinar!

NAFLDsym v2B Beta Release is Here: Insight into the Next Gen QSP Software Tool in Julia with Blazing Speed and Updated Interfacing
Join us in this webinar to learn about the technical advances made to the NAFLDsym platform in the latest release: version 2B Beta.

What’s New in GastroPlus® v9.8.3?
Learn about the new and improved functionality to drive advances to PBBM/PBPK applications from discovery through post-approval.

GastroPlus as an Educational Tool Teaching of Pharmacokinetics & Biopharmaceutics with PBPK Modeling
Students have an increased interest in learning physiologically based pharmacokinetic (PBPK) modeling, but this requires an in-depth understanding of biopharmaceutics and pharmacokinetics.
Project Optimus –FDA’s “New” Dose Optimization & Selection Paradigm in Oncology Drug Development
Dr. Brian P. Booth and Dr. Hao Zhu from the Office of Clinical Pharmacology (OCP) at the FDA will present and discuss Project Optimus, an initiative from the Oncology Center of Excellence (OCE) to reform the dose optimization and dose selection paradigm in oncology drug development.

Application of PBPK Modeling in Pediatric Drug Development (GastroPlus®)
Role of PBPK in Pediatric Drug Development

Next Gen Tech for QST/QSP & PBPK Modeling
Simulations Plus is excited to share some technical advances that have been made to two of the feature models developed by the DILIsym Services division!

Predicting PK of Multisource Acyclovir Oral Products Through PBBM Modeling
Highly variable disposition after oral ingestion of acyclovir has been reported, although little is known regarding the underlying mechanisms.

Using PBPK modeling to assess the impact of diseases on oral drug absorption: case study in HIV-infected patients
Randomized clinical trial is the most effective way to determine whether a cause-and-effect relationship exists between an intervention and a predefined clinical outcome.

ADMET Predictor® 10.4 (APX.4) Release Webinar
Eric Jamois, Director of Business Development, will host a webinar on Wednesday, May 18th to introduce the new ADMET Predictor® 10.4 (APX.4) flagship machine learning platform for ADMET modeling, with extended capabilities for data analysis, metabolism prediction, and AI-driven drug design. David Miller, VP of ADMET Cheminformatics will show some of the new features in APX.4.

Active Learning in a Pandemic: Teaching Biopharmaceutics with GastroPlus®
This webinar will showcase the use of GastroPlus® for a pharmacy course.

