Determining appropriate dosage for your first in human (FIH) clinical trials is critical.
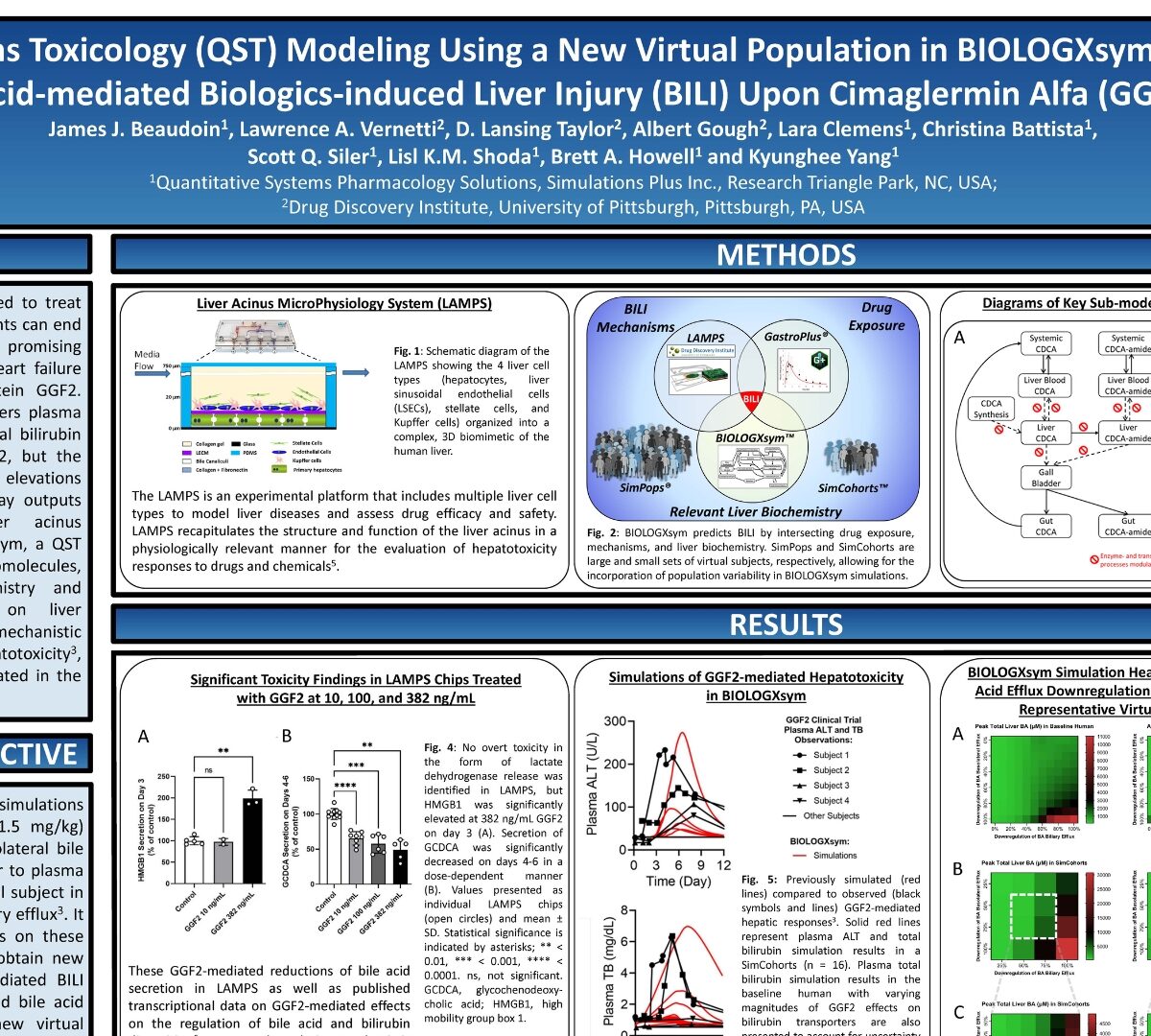
Quantitative Systems Toxicology (QST) Modeling Using a New Virtual Population in BIOLOGXsym Offers Mechanistic Insights Into Bile Acid-mediated Biologics-induced Liver Injury (BILI) Upon Cimaglermin Alfa (GGF2) Administration
Biopharmaceuticals are increasingly used to treat various medical conditions, but BILI events can end...
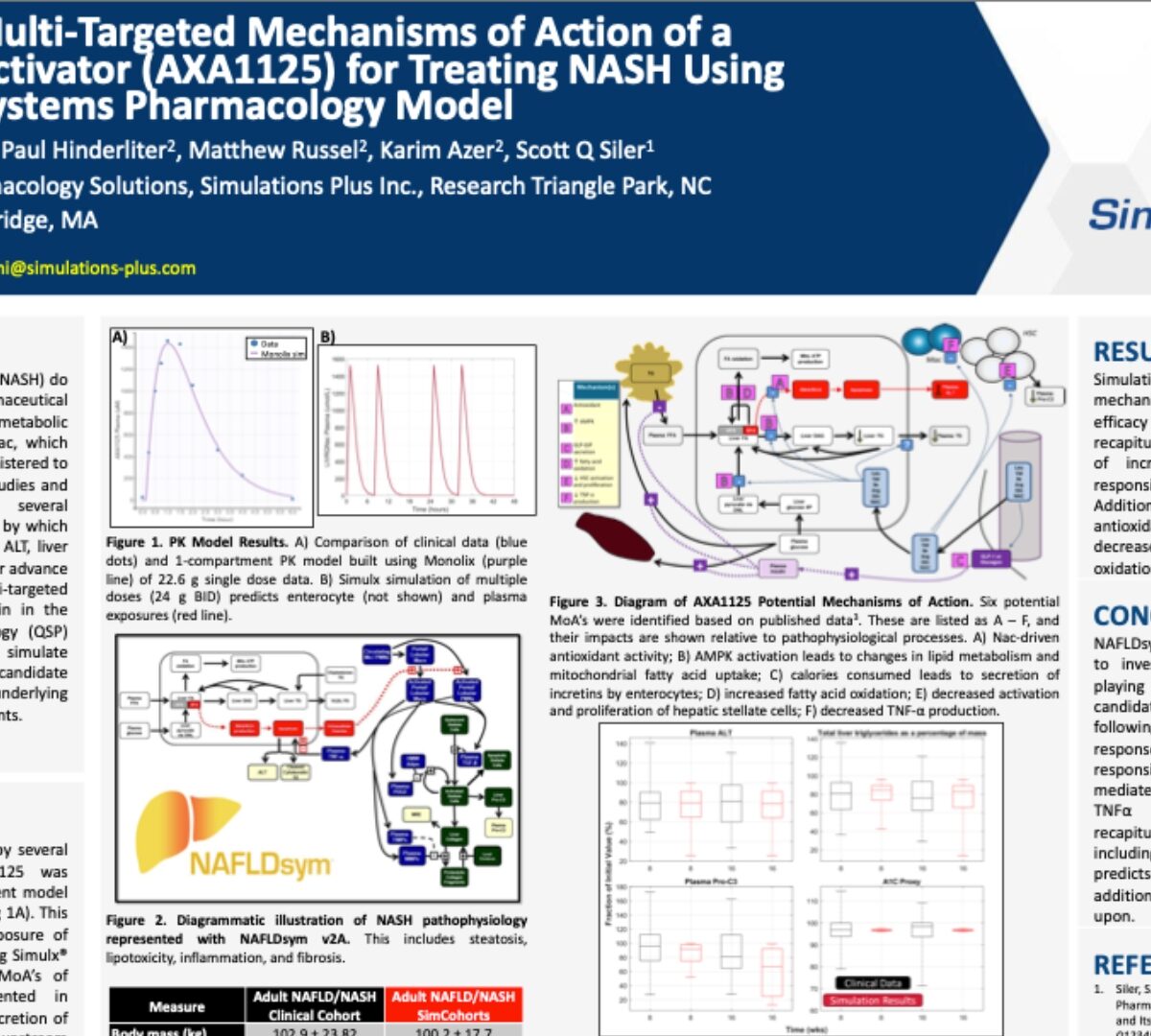
Advancing the Multi-Targeted Mechanisms of Action of a Mitochondrial Activator (AXA1125) for Treating NASH Using a Quantitative Systems Pharmacology Model
Patients with non-alcoholic steatohepatitis (NASH) do not currently have options for pharmaceutical...
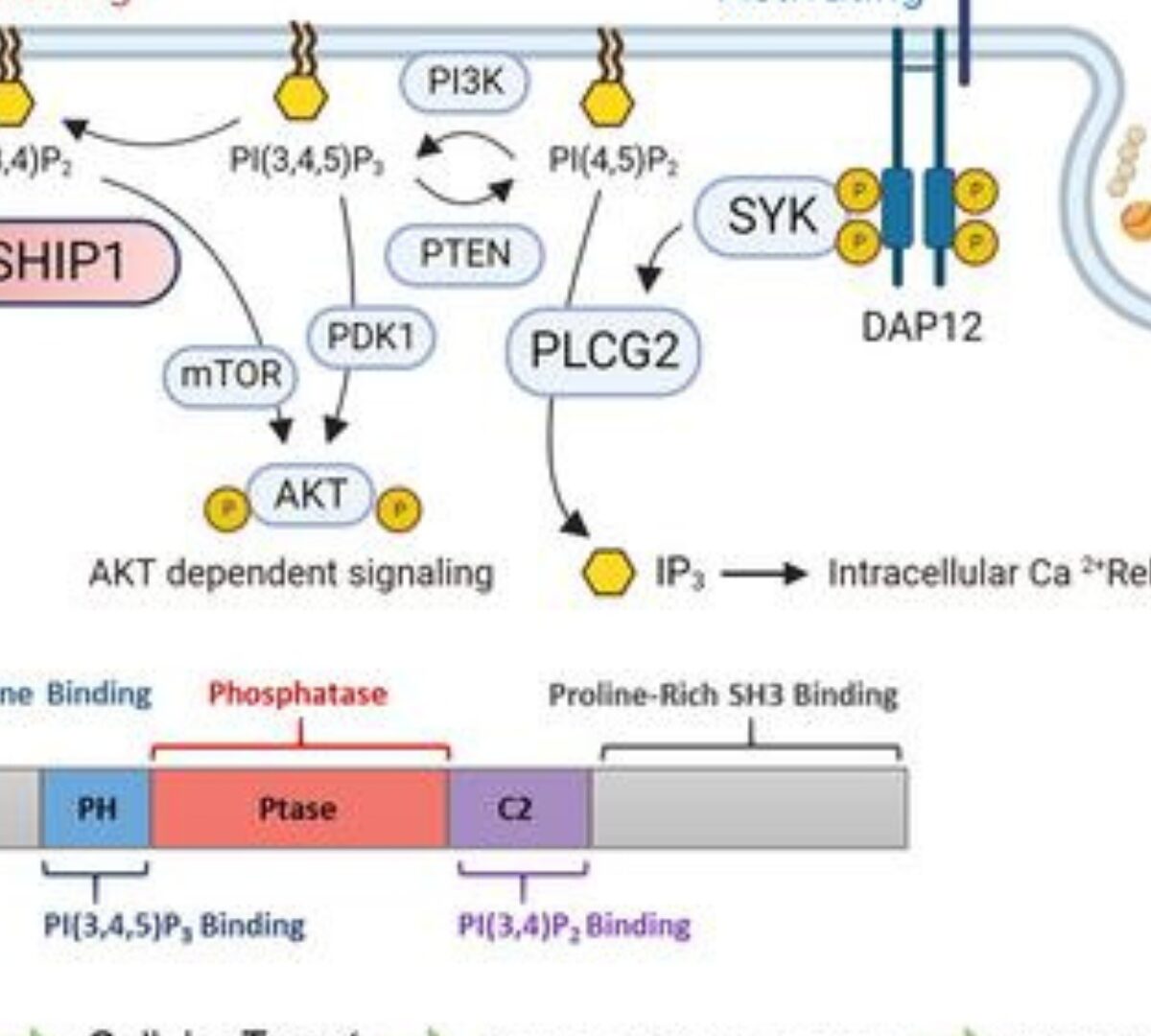
SHIP1 therapeutic target enablement: Identification and evaluation of inhibitors for the treatment of late-onset Alzheimer’s disease
The risk of developing Alzheimer's disease is associated with genes involved in microglial function.
Predicting the pharmacokinetics and pharmacodynamics of antisense oligonucleotides: an overview of various approaches and opportunities for PBPK/PD modelling
Advances in research and development (R&D) have enabled many approvals of antisense oligonucleotides (ASOs). Its administration expanded from systemic to local for treating various diseases, where predicting target tissue exposures and pharmacokinetics (PK) and pharmacodynamics (PD) in human can be critical.
Mechanistic dissolution data modeling for PBBM introduction: The P-PSD approach
Collect input data for dissolution modeling and test multiple conditions

November 2023 GastroPlus Newsletter
GastroPlus® Newsletter November 2023
Physiologically Based Pharmacokinetic modelling of drugs in pregnancy: A mini-review on availability and limitations
Physiologically based pharmacokinetic (PBPK) modelling in pregnancy is a relatively new approach that is increasingly being used to assess drug systemic exposure in pregnant women to potentially inform dosing adjustments.
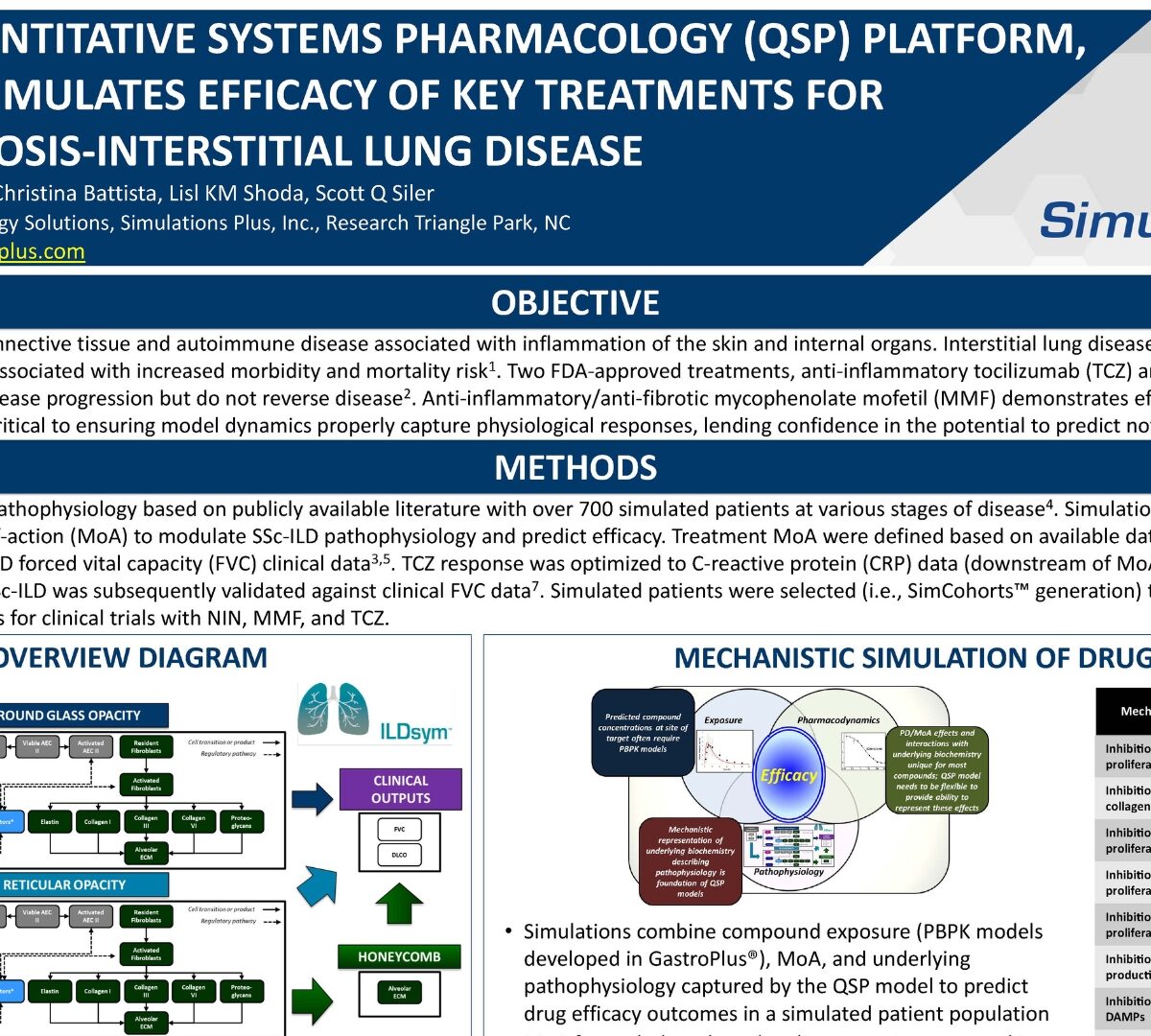
ILDSYM®, A Quantitative Systems Pharmacology (QSP) Platform, Successfully Simulates Efficacy of Key Treatments for Systemic Sclerosis-Interstitial Lung Disease
Systemic sclerosis (SSc) is a rare connective tissue and autoimmune disease associated with inflammation of the skin and internal organs. Interstitial lung disease (ILD), a frequent complication of SSc with highly variable course, is associated with increased morbidity and mortality risk¹. Two FDA-approved treatments, anti-inflammatory tocilizumab (TCZ) and anti-fibrotic nintedanib (NIN)...
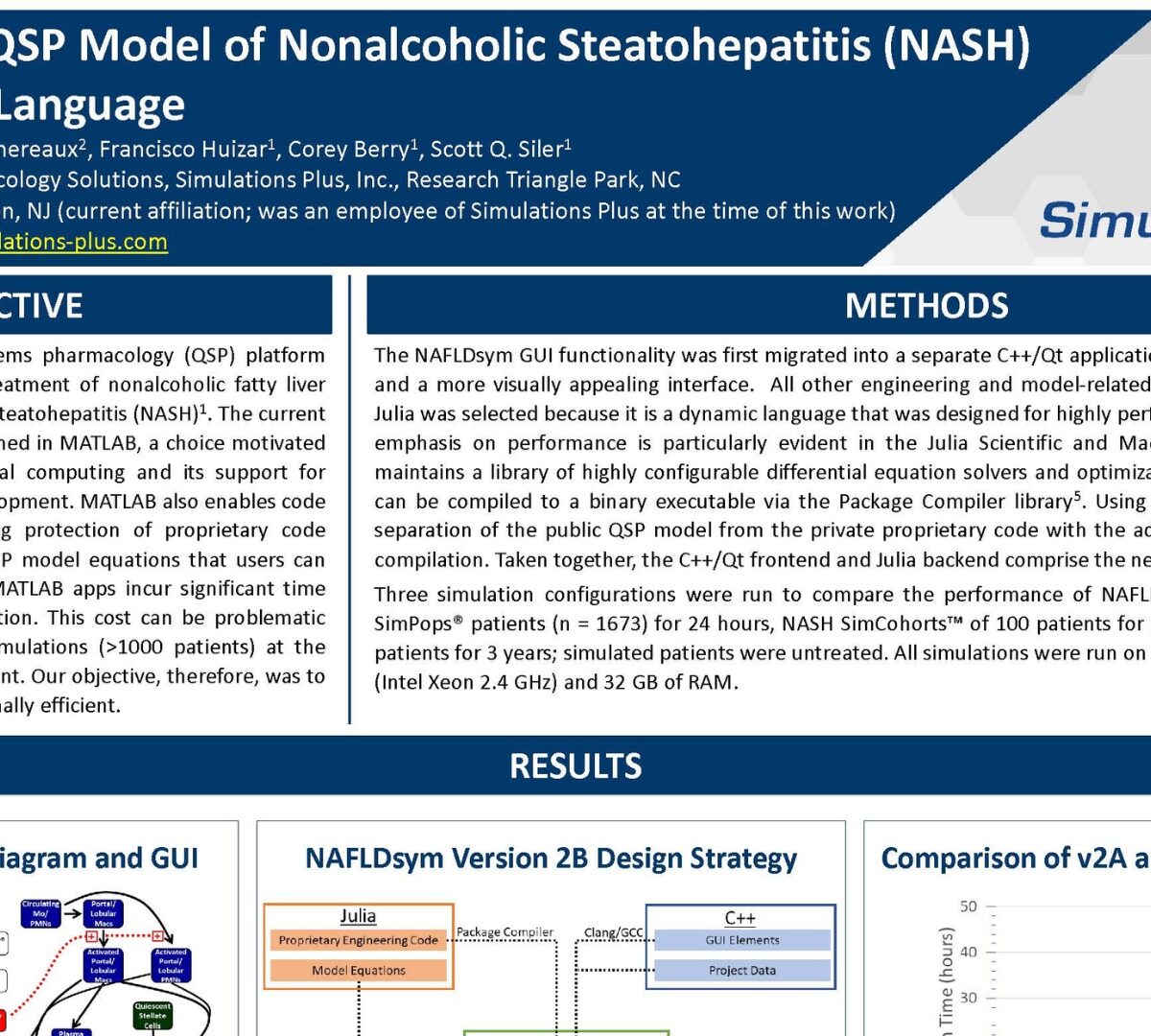
Accelerating a QSP Model of Nonalcoholic Steatohepatitis (NASH) Using the Julia Language
NAFLDsym® is a quantitative systems pharmacology (QSP) platform that simulates progression and treatment of nonalcoholic fatty liver...
Simulations Plus Investor Day 2023
Putting Clients First Drives Growth and Fuels Innovation

Ionization Prediction Summit Webinar Series: Session 4 Experimental Aspects of pKa Measurements
Multiprotic ionization is often poorly understood in the pharmaceutical industry.
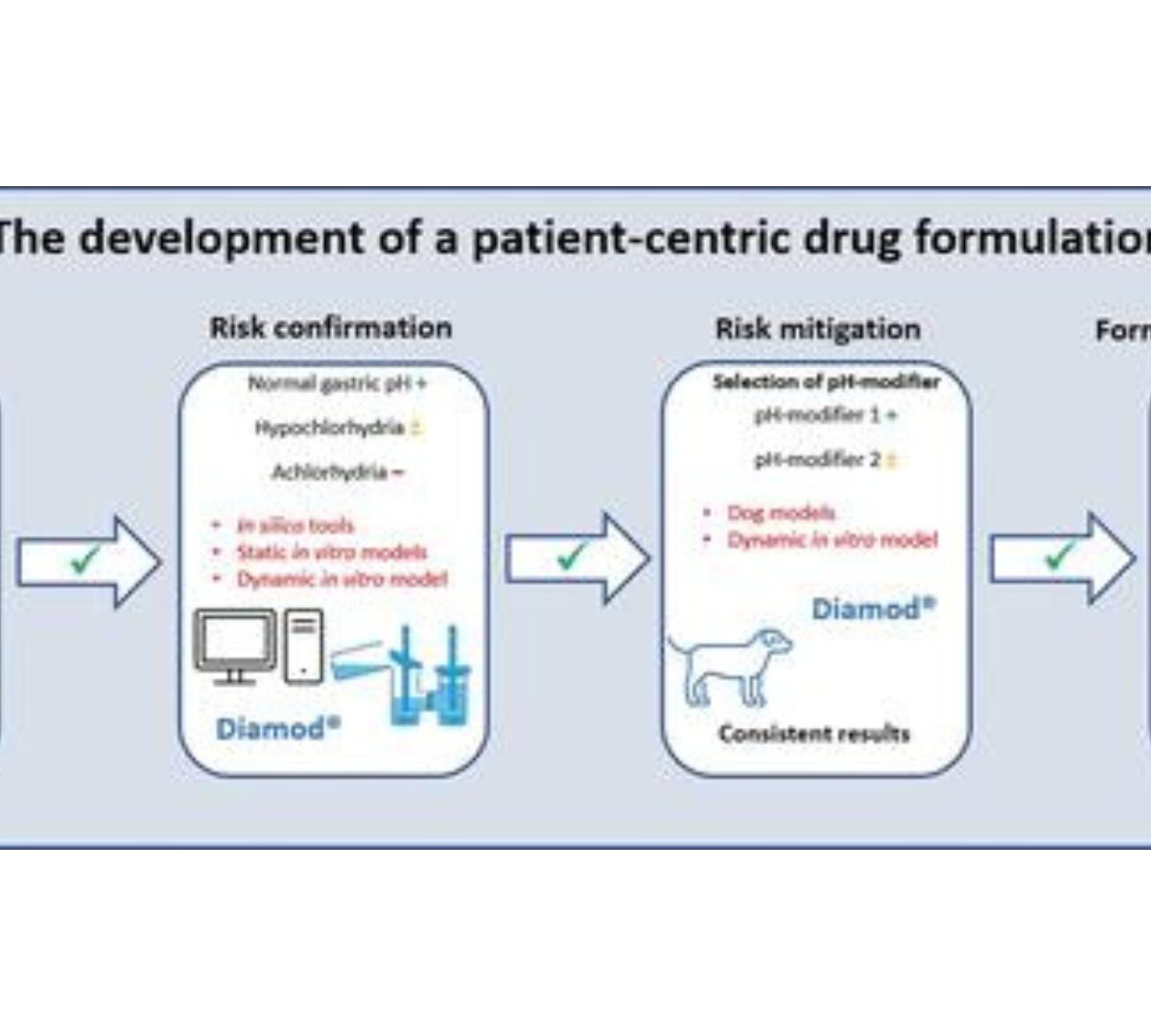
Contribution of the Dynamic Intestinal Absorption Model (Diamod) to the Development of a Patient-Centric Drug Formulation
Compound X is a weak basic drug targeting the early stages of Parkinson’s disease, for which a theoretical risk assessment has indicated that elevated gastric pH conditions could potentially result in reduced plasma concentrations.

Mechanistic Representation of NAG Release in Relation to Renal Proximal Tubular Cellular Injury
Novel Acute kidney injury (AKI) biomarkers enhance disease understanding and aid...
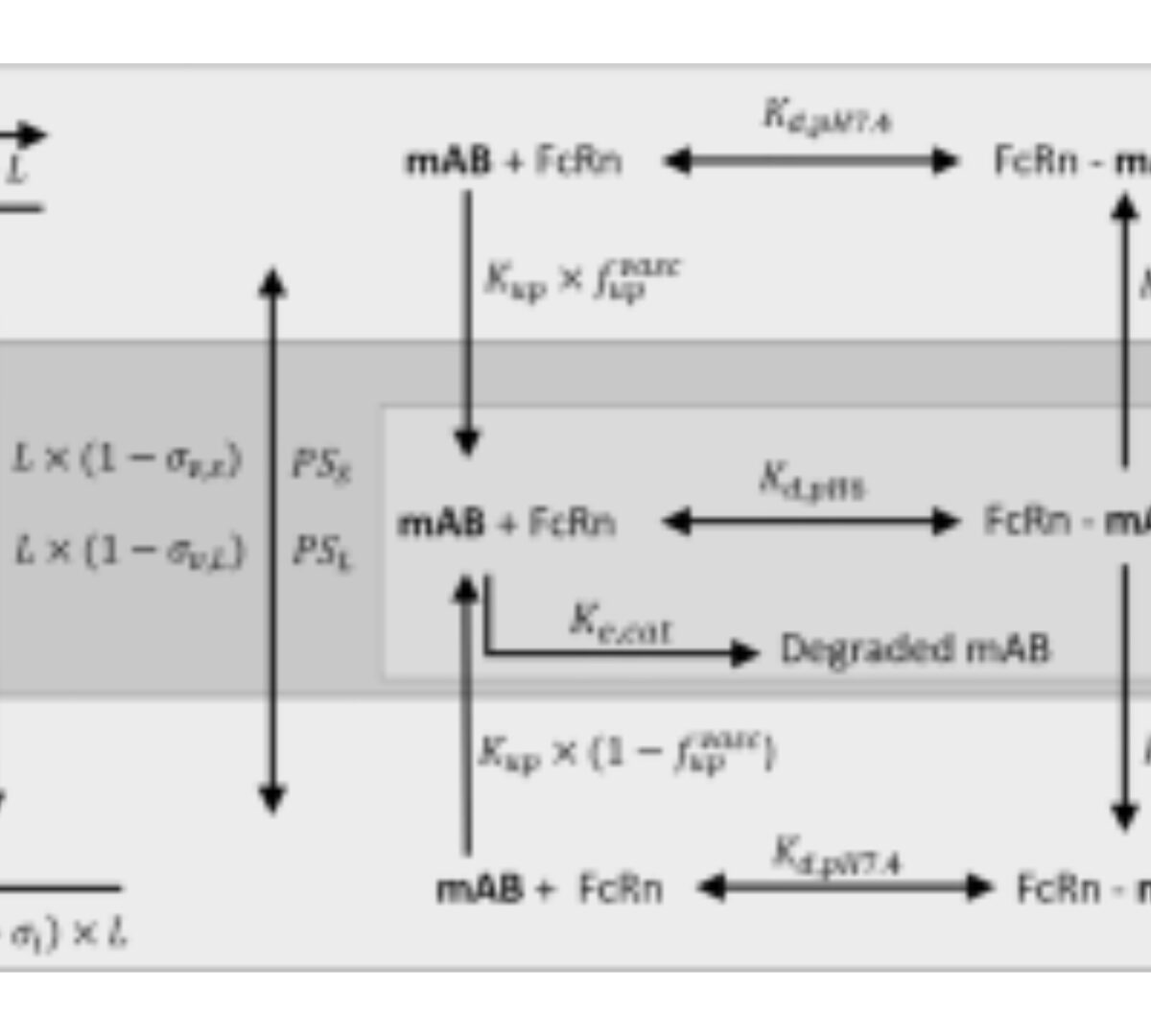
Comparison of Monoclonal Antibody Disposition Predictions Using Different Physiologically Based Pharmacokinetic Modelling Platforms
Physiologically based pharmacokinetic (PBPK) models can be used to leverage physiological and in vitro data to predict monoclonal antibody (mAb) concentrations in serum and tissues.
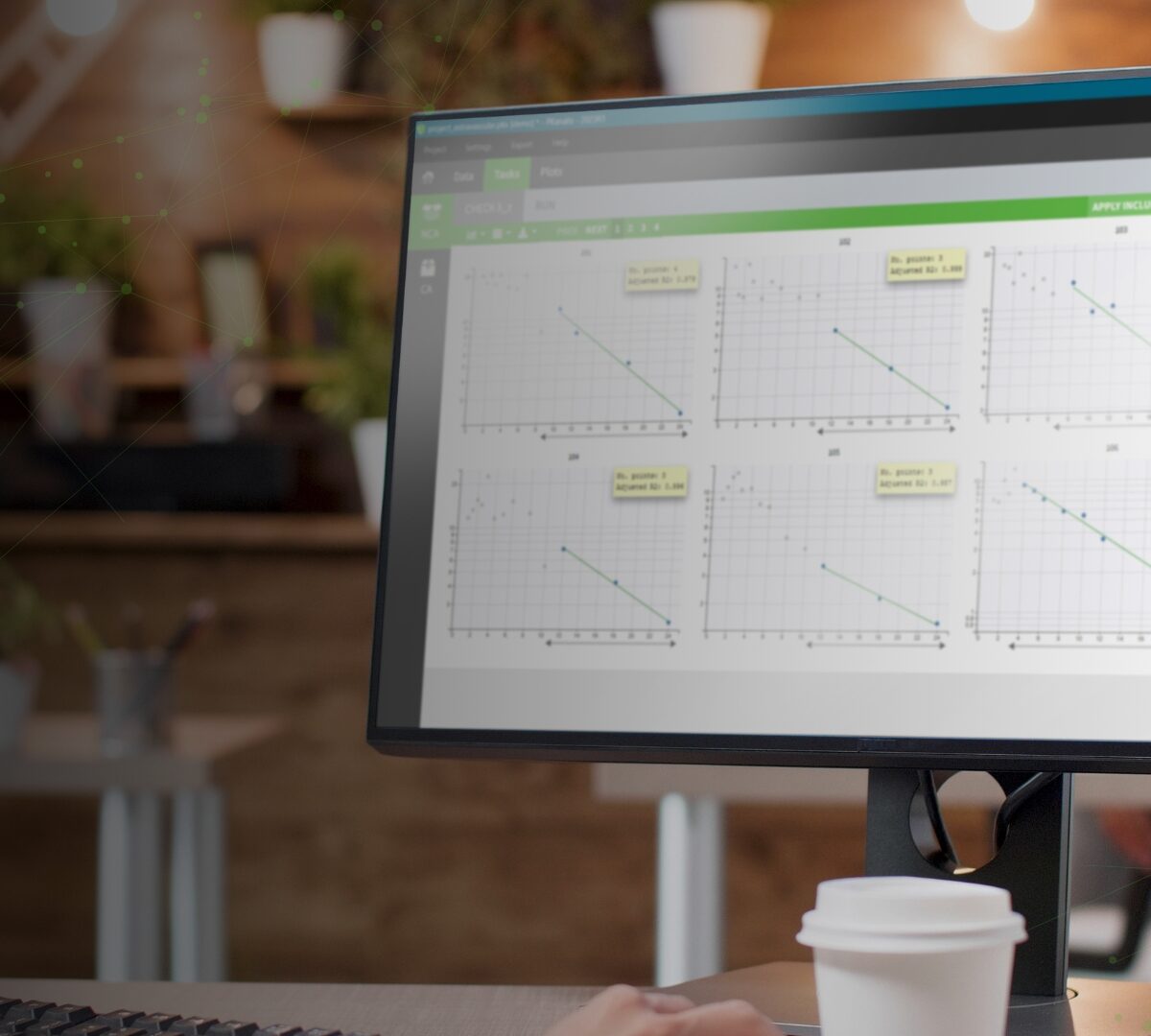
PKanalix Platform Comparision
PKanalix® is a user-friendly and fast application for compartmental analysis (CA), non-compartmental analysis (NCA) and bioequivalence studies (BE)
Use of In silico Methodologies to Predict the Bioavailability of Oral Suspensions: A Regulatory Approach
Oral suspensions are heterogeneous disperse systems, and the particle size distribution, crystalline form of the dispersed solid, and composition of the formulation can be listed as parameters that control the drug dissolution rate and its bioavailability.
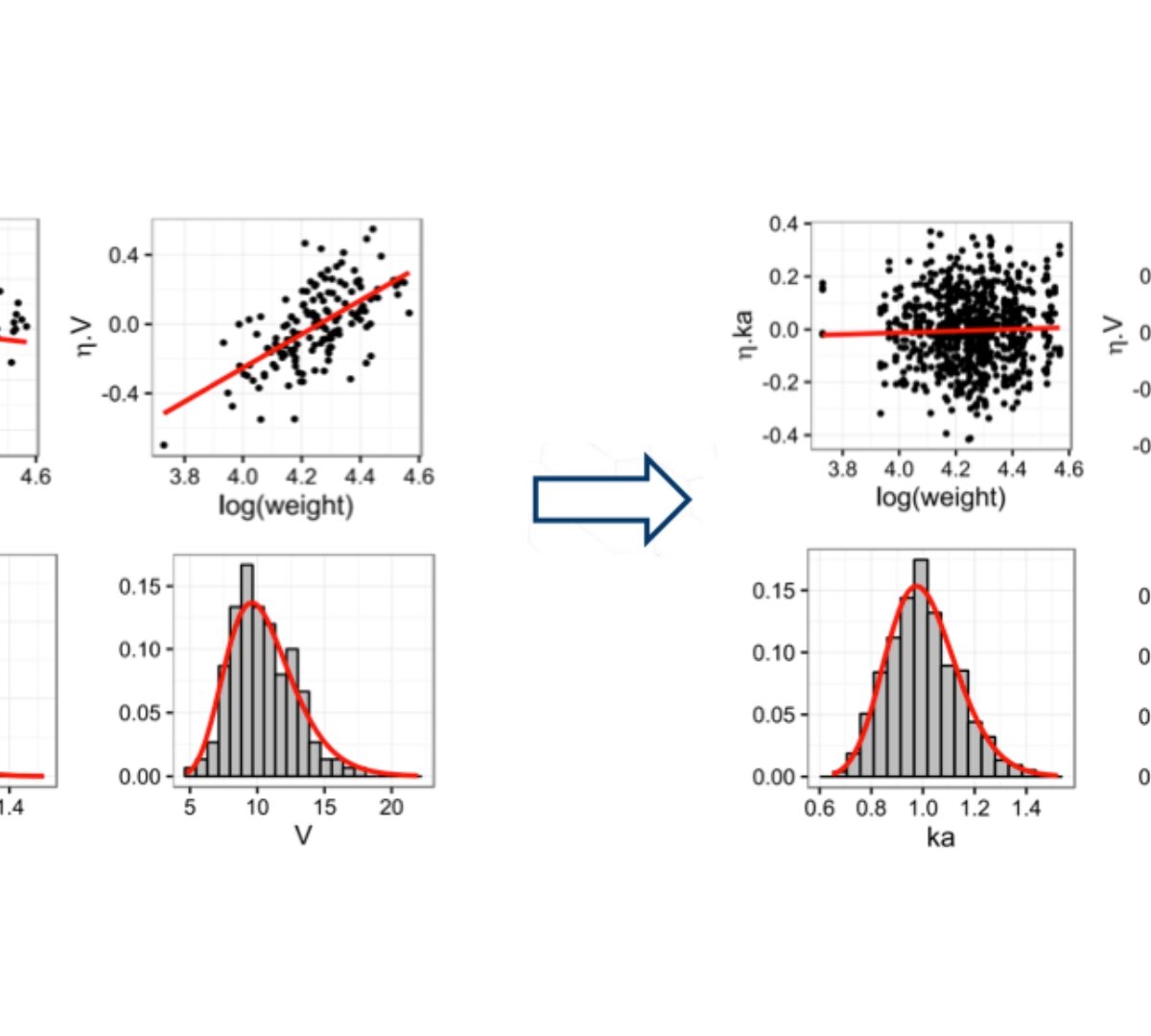
Automated covariate selection: SAMBA and COSSAC algorithms
COSSAC Conditional Sampling use for Stepwise Approach based on Correlation tests
SAMBA Stochastic Approximation for Model Building Algorithm
Voices in Molecular Pharmaceutics: Meet Dr. Bart Hens, A Sociable Scientist Focusing on Multidisciplinary Connections to Unravel the Gaps of Oral Drug Behavior in the Human Gastrointestinal Tract
Bart Hens (Pharm.D., Ph.D.) holds a Ph.D. degree in Pharmaceutical Sciences obtained from KU Leuven (Supervisor: Prof. Dr. Patrick Augustijns - Leuven, Belgium).