The aim of this study was to validate the utility of physiologically based pharamcokinetic (PBPK) models fore predictioin of DDI between cilostazol, kectoconazole, omeprazole and quindine.

Understanding the effect of API properties on bioavailability through absorption modeling
Selection of API phase is one of the first decision points in the formulation development process.

Simulations Plus Reports FY2008 Financial Results
Earnings Up 17.7%, Shareholder Equity Up 29.4% Over Previous Fiscal Year

Prediction of Drug Clearance by Glucuronidation from in Vitro Data: Use of Combined Cytochrome P450 and UDP-Glucuronosyltransferase Cofactors in Alamethicin-Activated Human Liver Microsomes
Glucuronidation via UDP-glucuronosyltransferase (UGT) is an increasingly important clearance pathway.

Toward an improved prediction of human in vivo brain penetration
The penetration of drugs into the central nervous system is a composite of both the rate of drug uptake across the blood–brain barrier and the extent of distribution into brain tissue compartments.
General Approach to Calculation of Tissue:Plasma Partition Coefficients for Physiologically Based Pharmacokinetic (PBPK) Modeling
To conduct a comprehensive evaluation of methods for calculation of tissue/plasma partition coefficients with a focus on correct prediction of volume of distribution and recommendation for a general…

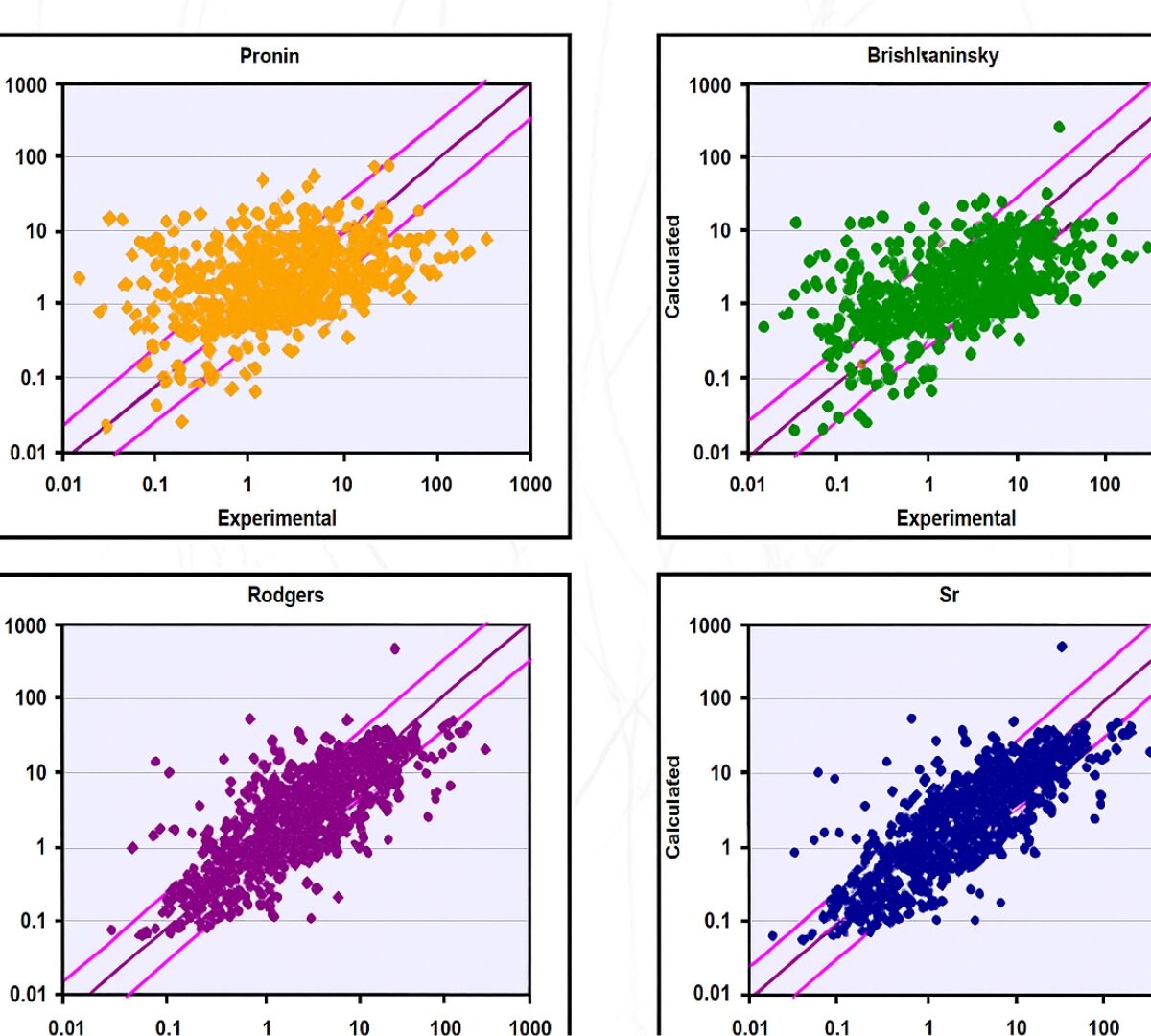
Role of Fraction Unbound in Plasma in Calculations of Tissue:Plasma Partition Coefficients
Previous investigations have shown that the Rodgers and Rowland method [Rodgers 2007] for prediction of tissue:plasma partition coefficients (Kps) provides good prediction for compounds with low to moderate…

Level A IVIVC Using a Comprehensive Absorption/PBPK Model for Metoprolol
Wagner-Nelson, Loo-Riegelman, numerical deconvolution, and convolution-based methods are conventional ways to form an in vitro-in vivo correlation (IVIVC). The ultimate goal for forming an IVIVC is to…

Beauty and the Beast?
Several large Pharma companies have announced interest in acquiring small biotech companies. Many Pharma companies have reduced or eliminated drug discovery efforts, and with stock prices back at 2003 levels, there certainly is a great deal of sense in these acquisitions. But finding another way to integrate these companies and their development portfolio also makes a great deal of sense.

John Muir (1838 to 1914) and advice for surviving the Economic Crisis of 2008
John Muir was one of the first climbers to explore and climb many of the peaks in Yosemite Valley in California’s High Sierra. During his first ascent of Mount Ritter in 1872, he became gripped with fear.

Kerfuffle! (pt 1)
A kerfuffle is the polite term for a cascading series of errors that can be initiated by a seemingly innocuous event that then leads to other errors that seem to gain in severity and impact. Kerfuffles can appear in any line of work or play that involves a linked series of tasks with downstream implications. In fact, the modeling and simulation activities performed to support model-based drug development have the potential to produce a catalogue of kerfuffles that can culminate in the failure to deliver modeling and simulation results when they are needed for decision-making. Kerfuffles often have their origins in inadvertent oversights committed early in the study design and data collection process or in the commonplace shortcuts taken to deliver preliminary (“quick-and-dirty”) results for internal use.

Simulations Plus Announces Share Repurchase Program
Up to $2.5 million over 12 months authorized by board of directors

Modeling Effects of Exenatide on the Pharmacokinetics of Acetaminophen, Digoxin, and Warfarin
Exenatide, a 39-amino acid peptide used for treatment of type 2 diabetes, is known to inhibit gastric emptying and as a result to alter the absorption of orally administered concomitant medications.

Simulations Plus Signs Another Collaboration Agreement
Companies to Cooperate on Development of Advanced Ocular Delivery Simulation

Pharmacokinetic/pharmacodynamic modeling and simulation of neutropenia during phase I development of liposome-entrapped paclitaxel
To evaluate the maximum tolerated dose (MTD), dose-limiting toxicities (DLT), and pharmacokinetics of liposome-entrapped paclitaxel...

Structural Requirements for Drug Inhibition of the Liver Specific Human Organic Cation Transport Protein 1
The liver-specific organic cation transport protein (OCT1; SLC22A1) transports several cationic drugs including the antidiabetic drug metformin and the anticancer agents oxaliplatin and imatinib.

U.S. FDA Licenses ADMET Predictor™ from Simulations Plus
Adds Property Prediction Software to Earlier GastroPlus(™) Licenses

U.S. National Institutes of Health Renews ClassPharmer Software Licenses
Chemical Genomics Center Renews Five Licenses
