Deficiency or dysfunction in regulatory factors that control the complement cascade critically contribute to several rare diseases...

Physiologically Based Biopharmaceutics Modeling (PBBM): Best Practices for Drug Product Quality, Regulatory and Industry Perspectives: 2023 Workshop Summary Report
Physiologically based biopharmaceutics modeling (PBBM) is used to elevate drug product quality by providing a more accurate and holistic understanding of how drugs interact with the human body.

Development and application of the physiologically-based toxicokinetic (PBTK) model for ochratoxin A (OTA) in rats and humans
Ochratoxin A (OTA) is a common fungal toxin frequently detected in food and human plasma samples.

Exploring Lead-Like Molecules of Traditional Chinese Medicine for Treatment Quest against Aliarcobacter butzleri: In Silico Toxicity Assessment, Dynamics Simulation, and Pharmacokinetic Profiling
Aliarcobacter butzleri is a Gram-negative, curved or spiral-shaped, microaerophilic bacterium and causes human infections, specifically diarrhea, fever, and sepsis.

Validation of a Caco-2 microfluidic Chip model for predicting intestinal absorption of BCS Class I-IV drugs
Oral delivery is considered the most patient preferred route of drug administration, however, the drug must be sufficiently soluble and permeable to successfully formulate an oral formulation.

Predictions of tissue concentrations of myclobutanil, oxyfluorfen, and pronamide in rat and human after oral exposures via GastroPlusTM physiologically based pharmacokinetic modelling
Heritage agrochemicals like myclobutanil, oxyfluorfen, and pronamide, are extensively used in agriculture, with well-established studies on their animal toxicity.

Best of Both Worlds: An Expansion of the State of the Art pKa Model with Data from Three Industrial Partners
In a unique collaboration between Simulations Plus and several industrial partners, we were able to develop a new version 11.0 of the previously published1 in silico pKa model, S+pKa, with considerably improved prediction accuracy.

U.S. FDA Renews DILIsym® Software Licenses for 7th Year
Predicting DILI risk supports informed decision-making regarding drug evaluations and approvals

Simulations Plus Reports Second Quarter Fiscal 2024 Financial Results
Total revenue of $18.3 million and diluted earnings per share (EPS) of $0.20

April 2024 GastroPlus Newsletter
GastroPlus® Monthly Newsletter 2024
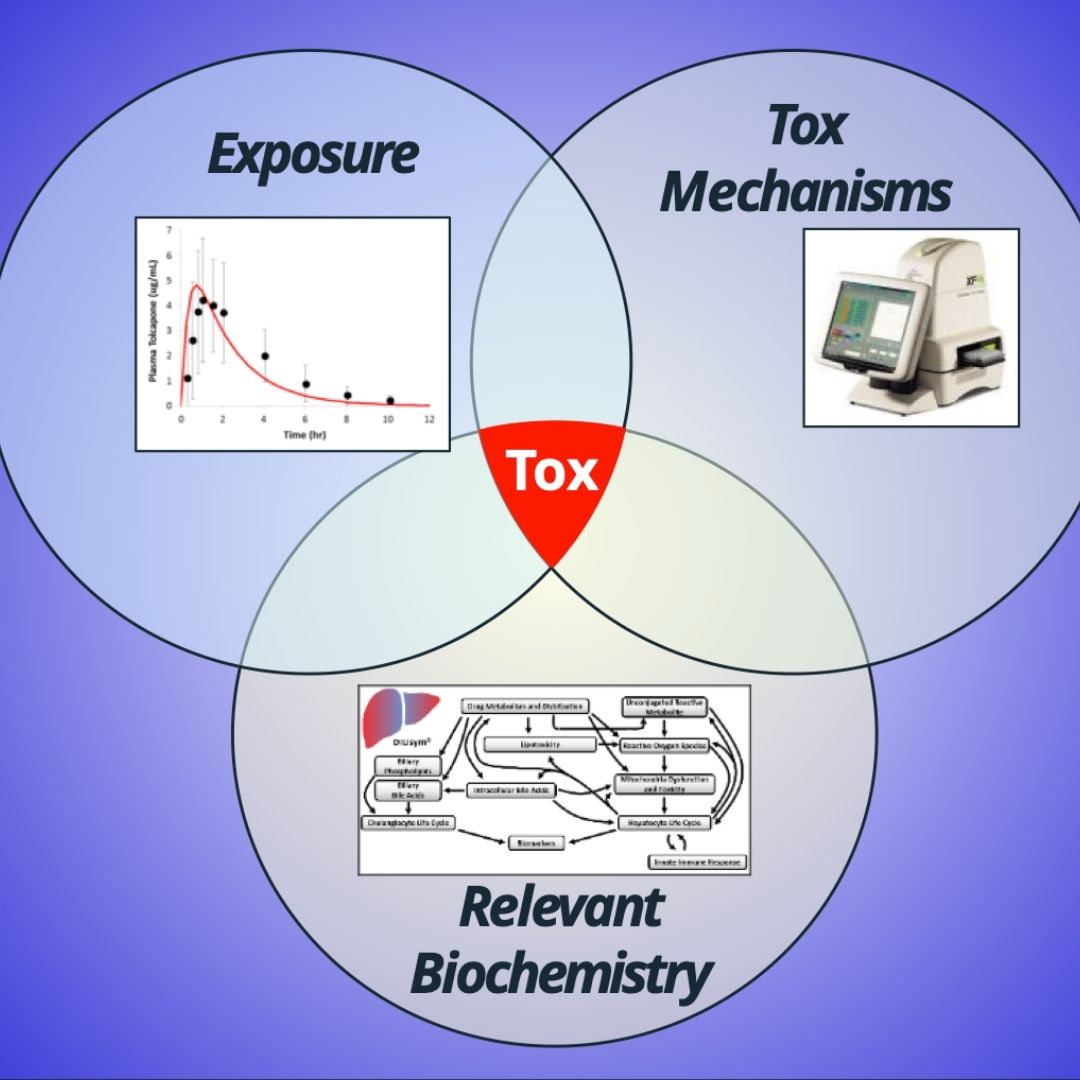
Construction of a Simulated Population of Post-Menopausal Women for the Prediction of Drug-Induced Liver Injury (DILI)
ASCPT 2024: Construction of a Simulated Population of Post-Menopausal Women for the Prediction of Drug-Induced Liver Injury (DILI)

A Biomarker-Focused QSP Model of Complement Alternative and Terminal Pathways to Evaluate Potential Targets for Therapeutic Impact in Complement-Associated Diseases: Paroxysmal Nocturnal Hemoglobinuria (PNH) as a Case Study
Complement overactivity has been implicated in multiple diseases, including PNH...

Optimizing Obesity Treatments: 3 Ways to Accelerate Your Program
As a nutritional biochemist by training, I am really excited about the effectiveness of current treatments for obese patients—and it extends well beyond weight loss.

Characterization of Pediatric Rectal Absorption, Drug Disposition, and Sedation Level for Midazolam Gel Using Physiologically Based Pharmacokinetic/Pharmacodynamic Modeling
This study aims to explore and characterize the role of pediatric sedation via rectal route.

Framework for Classifying Chemicals for Repeat Dose Toxicity using NAMs
Initially all chemicals are of High concern. Reassessment is based on accumulating evidence to potentially move chemicals to Medium or Low concern.

The bioequivalence study design recommendations for immediate-release solid oral dosage forms in the international pharmaceutical regulators programme participating regulators and organisations: differences and commonalities
Bioequivalence (BE) studies are considered the standard for demonstrating that the performance of a generic drug product in the human body is sufficiently similar to that of its comparator product.

International Perspectives on Model-Informed Precision Dosing: From the Data to the Patients
Identifying the right dose of the right drug for the right patient is a central objective of pharmaceutical drug development.

QSP in the Real World: Where Has Quantitative Systems Pharmacology (QSP) Been Used to Support Drug Development and With What Impact?
Quantitative systems pharmacology (QSP) is gaining acceptance and utilization in many drug development programs, but even those who appreciate its value may not fully recognize the extent to which it has already achieved impact and/or its range of potential applications.

Unlocking the Power of PBPK Modeling: PBPK for First-in-Human and Beyond
The mechanistic translation of nonclinical pharmacokinetic data to humans can make or break the success of your clinical plan.
