Understand how PBBM mechanistically integrates formulation dependent processes—such as dissolution, precipitation, gastrointestinal transit, and metabolism—to simulate oral drug absorption under varying prandial conditions.
AI-Driven Knowledge Management in PBPK Modeling: Challenges & Opportunities
High-quality data is the foundation of every major AI advance
The Impact of Simulation-Based Learning on Study Acceleration: Spoken from the Sponsor who Converted
Enhancing Training to be a personalized, representative and interactive strategic asset
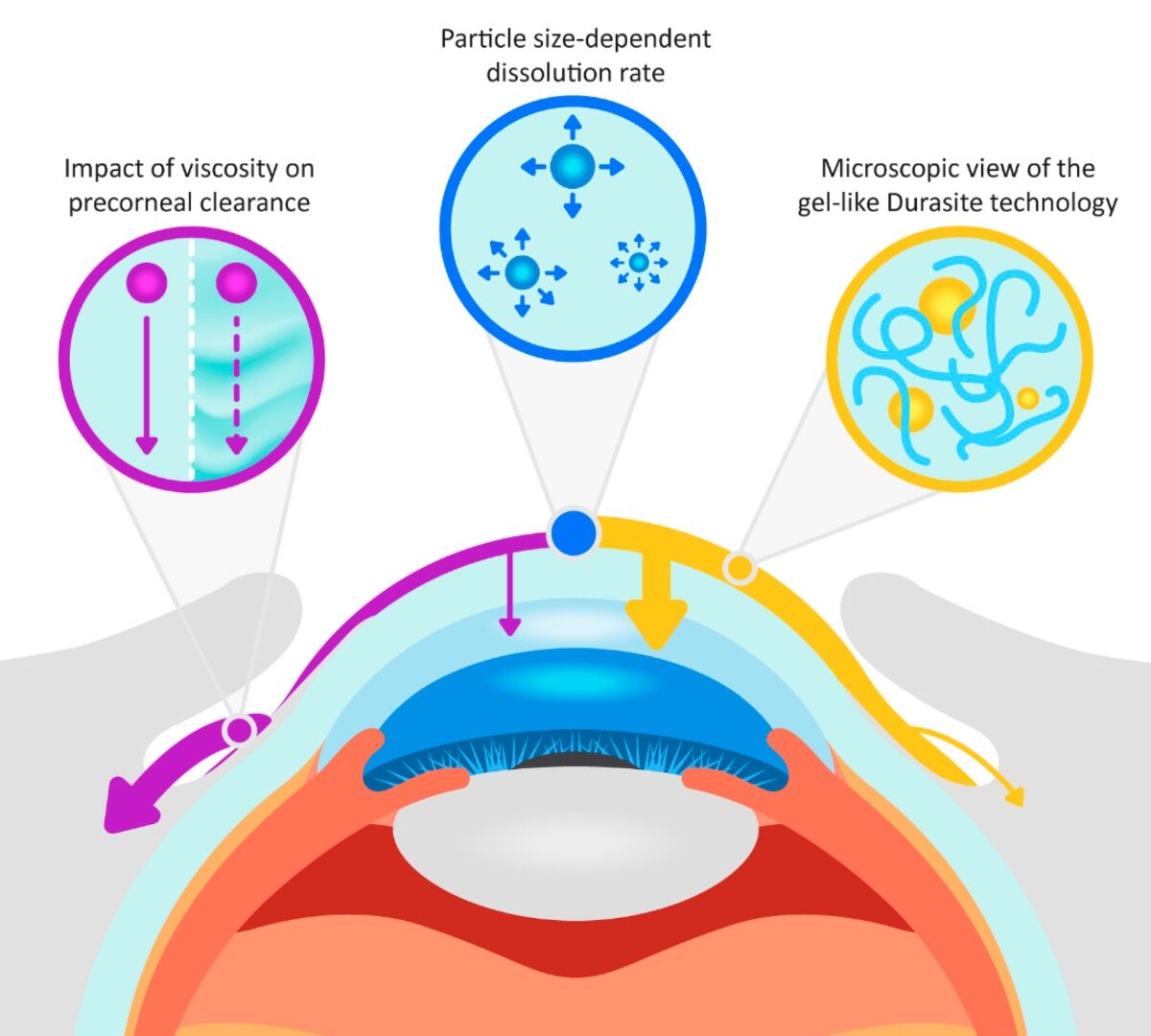
Basics of PBPK Modelling and Introduction to GastroPlus® X & its integration with AI
Overview of Mechanistic Processes in GastroPlus®
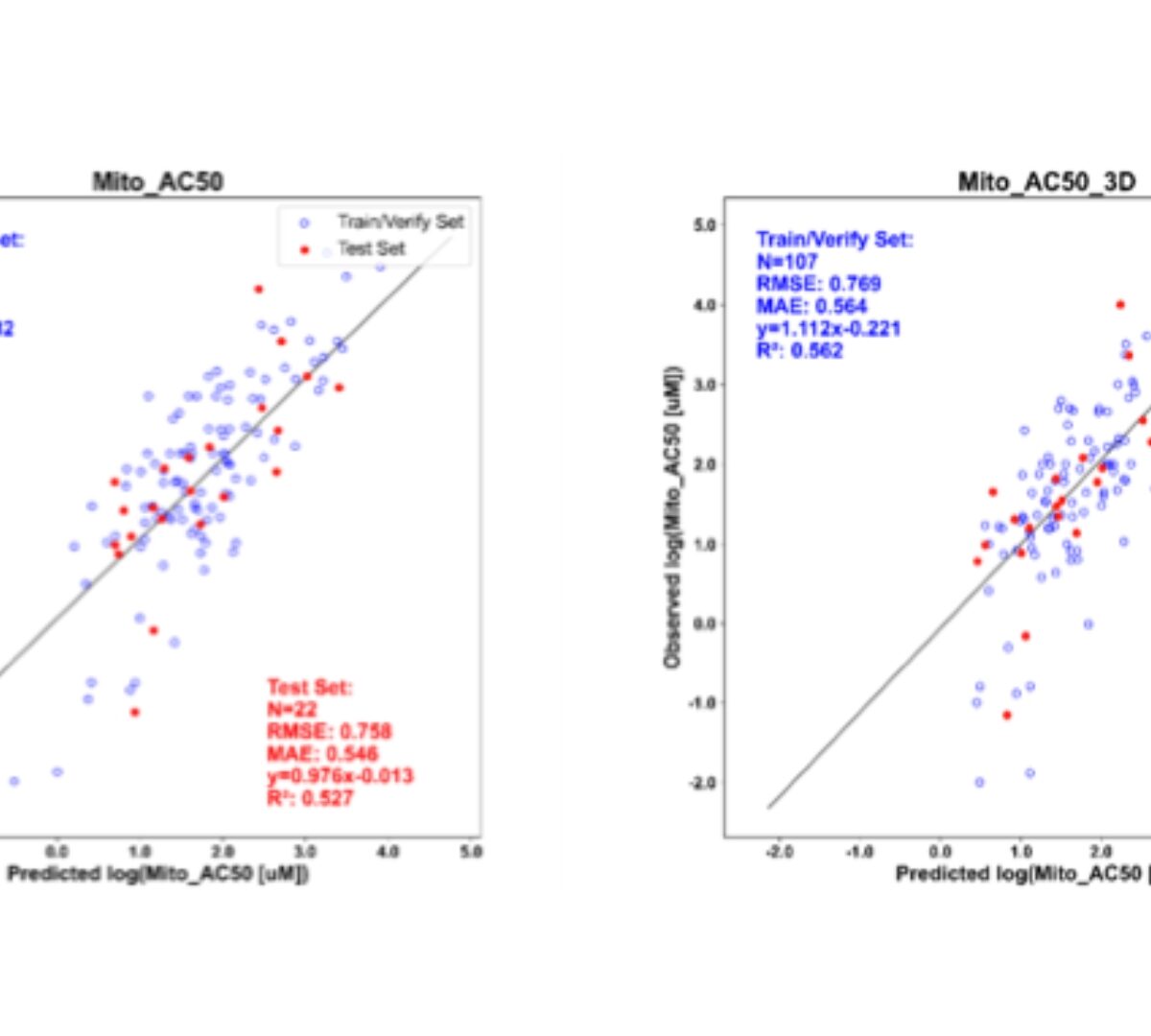
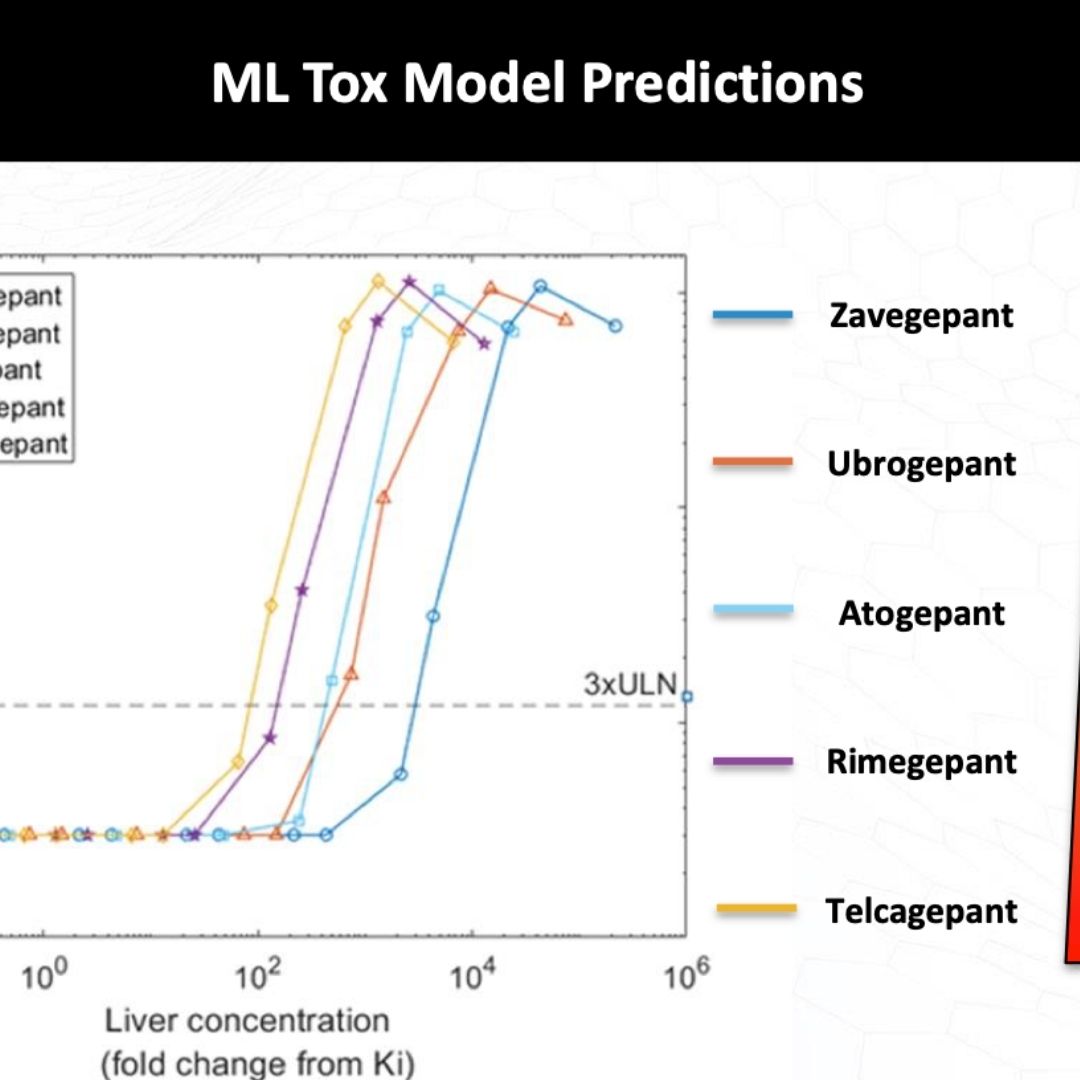
Application of Quantitative Systems Toxicology and Machine Learning Models in the Assessment of Drug-Induced Liver Injury
The QST Model DILIsym Provides More Comprehensive Predictions of DILI Risk than Artificial Intelligence Models
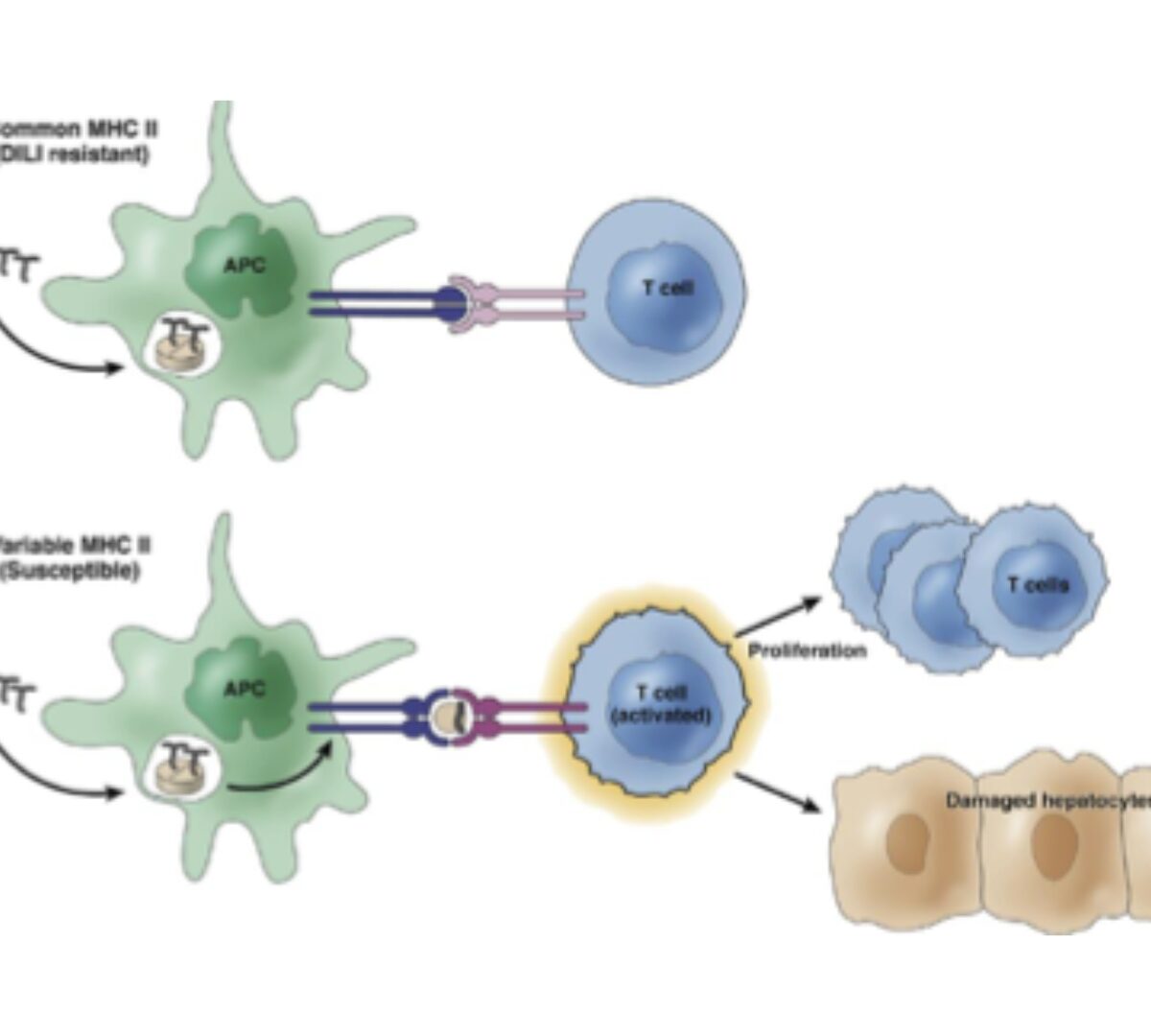
Successes and Failures of DILIsym
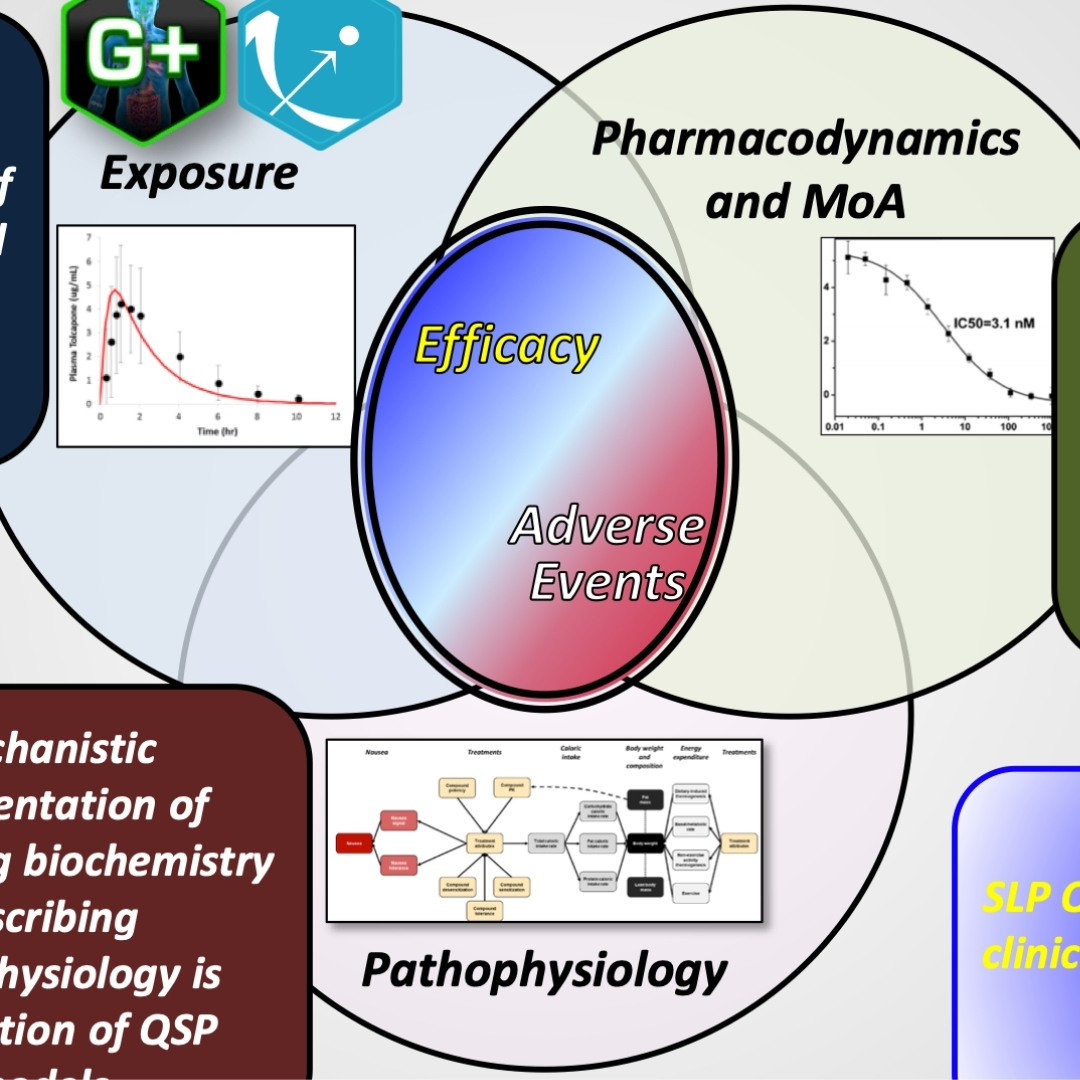
QST Models Predict Tox via the Intersection Between Exposure, Mechanisms, and Inter-Patient Variability
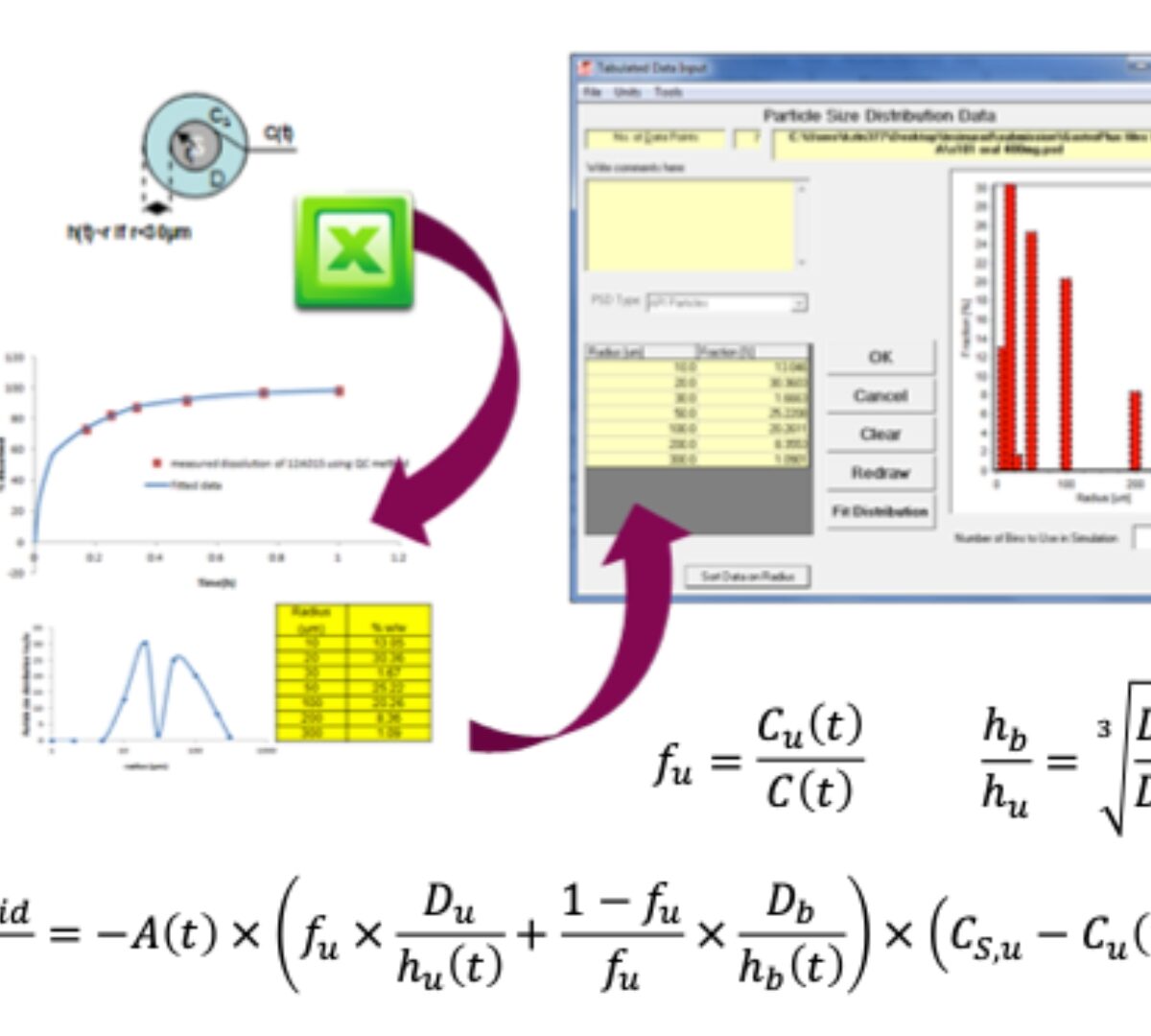
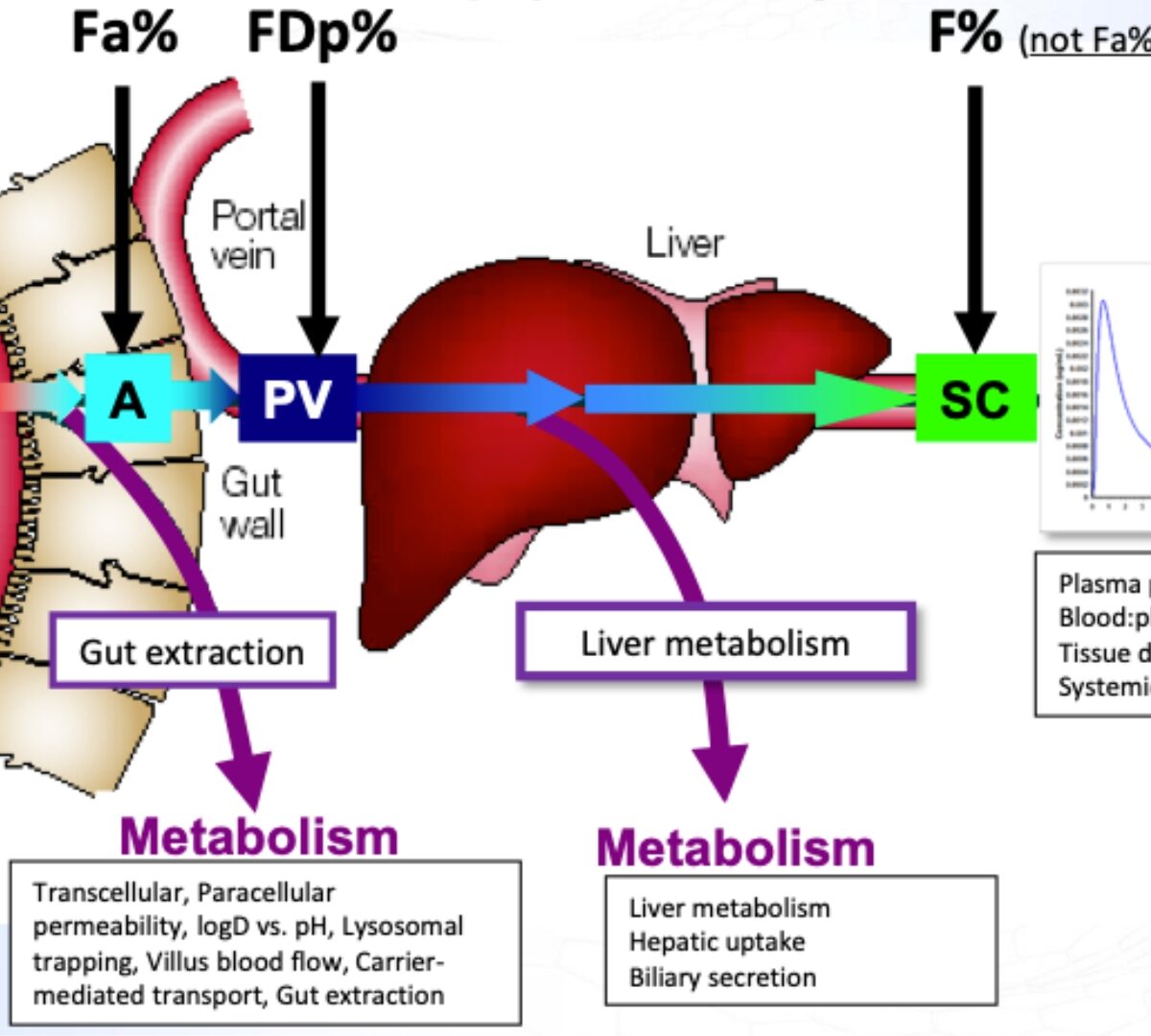
When Mechanistic IVIVC Falls Short: The Case for IVIVR in Oral Drug Delivery
Traditional IVIVC is robust for ER formulations where dissolution is rate limiting.
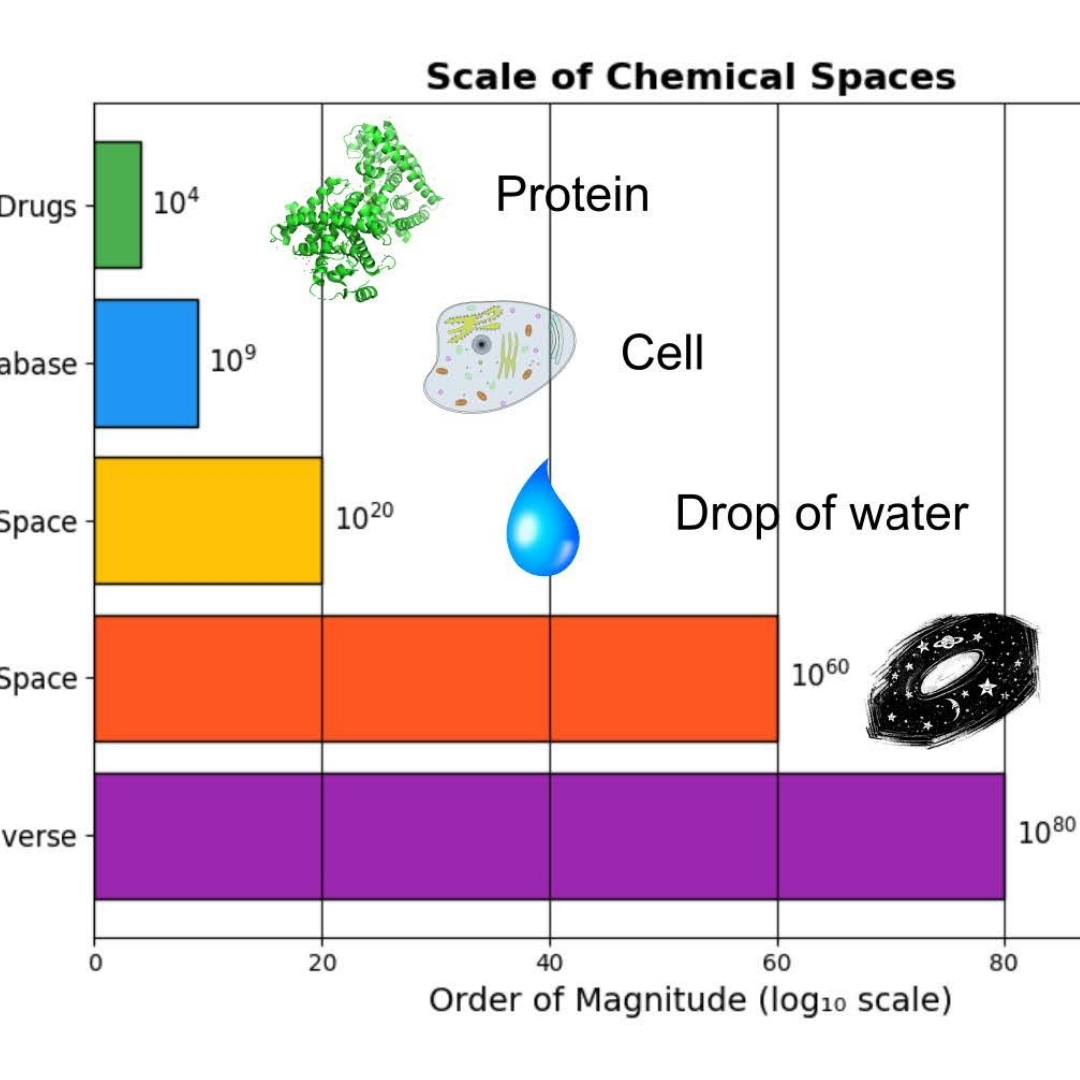
Navigating Synthon Space: Property-Driven Molecular Optimization for Pharmacokinetics
The Complexity of Chemical Space

Practical tips for using GenAI in Biomedicine
Now that we a are couple of years into the age of generative AI, it’s clear that it is transforming scientific research and drug discovery.
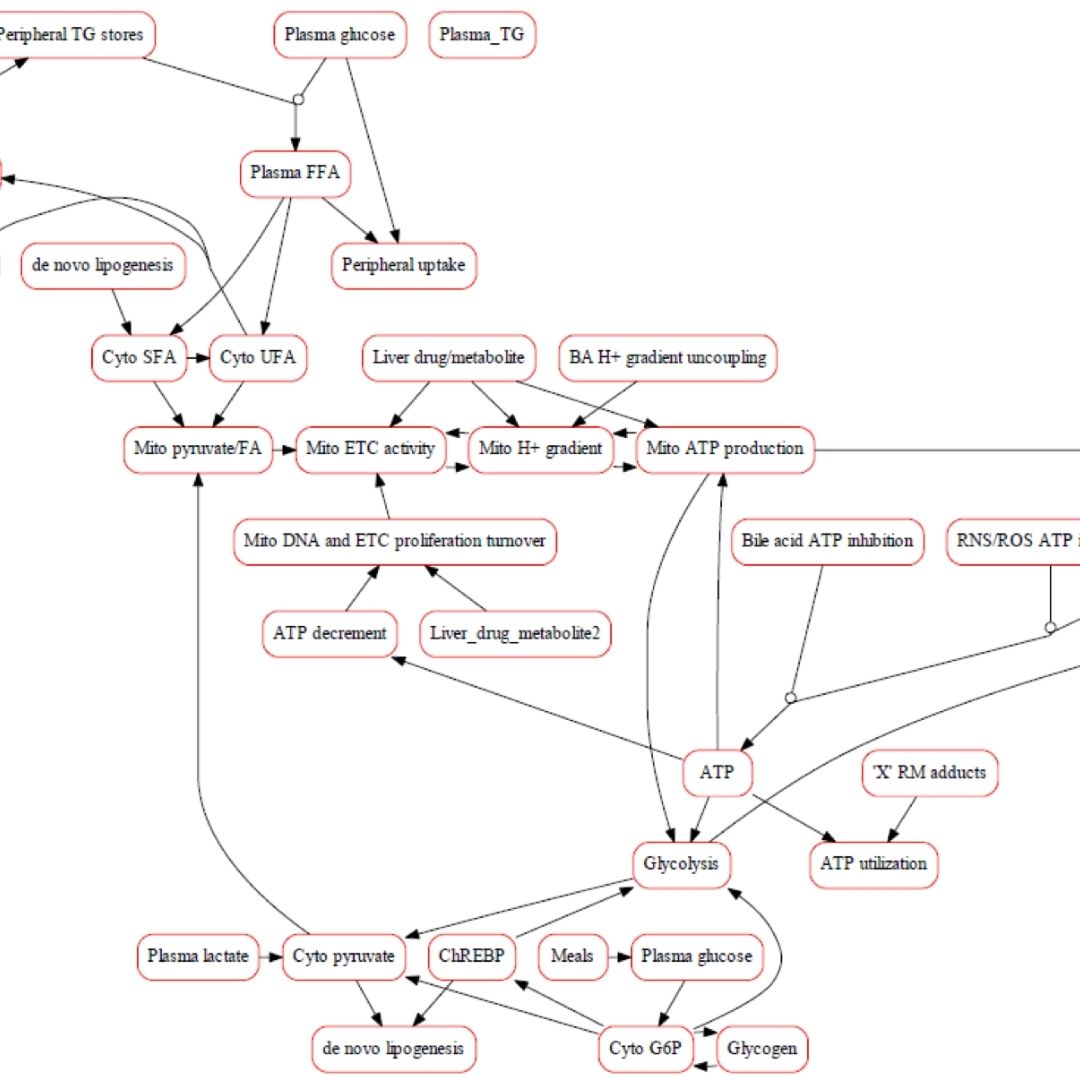
Integrating Human Biomimetic Liver Microphysiology System with Quantitative Systems Toxicology Modeling to Predict DILI
Integrating Human Biomimetic Liver Microphysiology System with Quantitative Systems Toxicology Modeling to Predict DILI
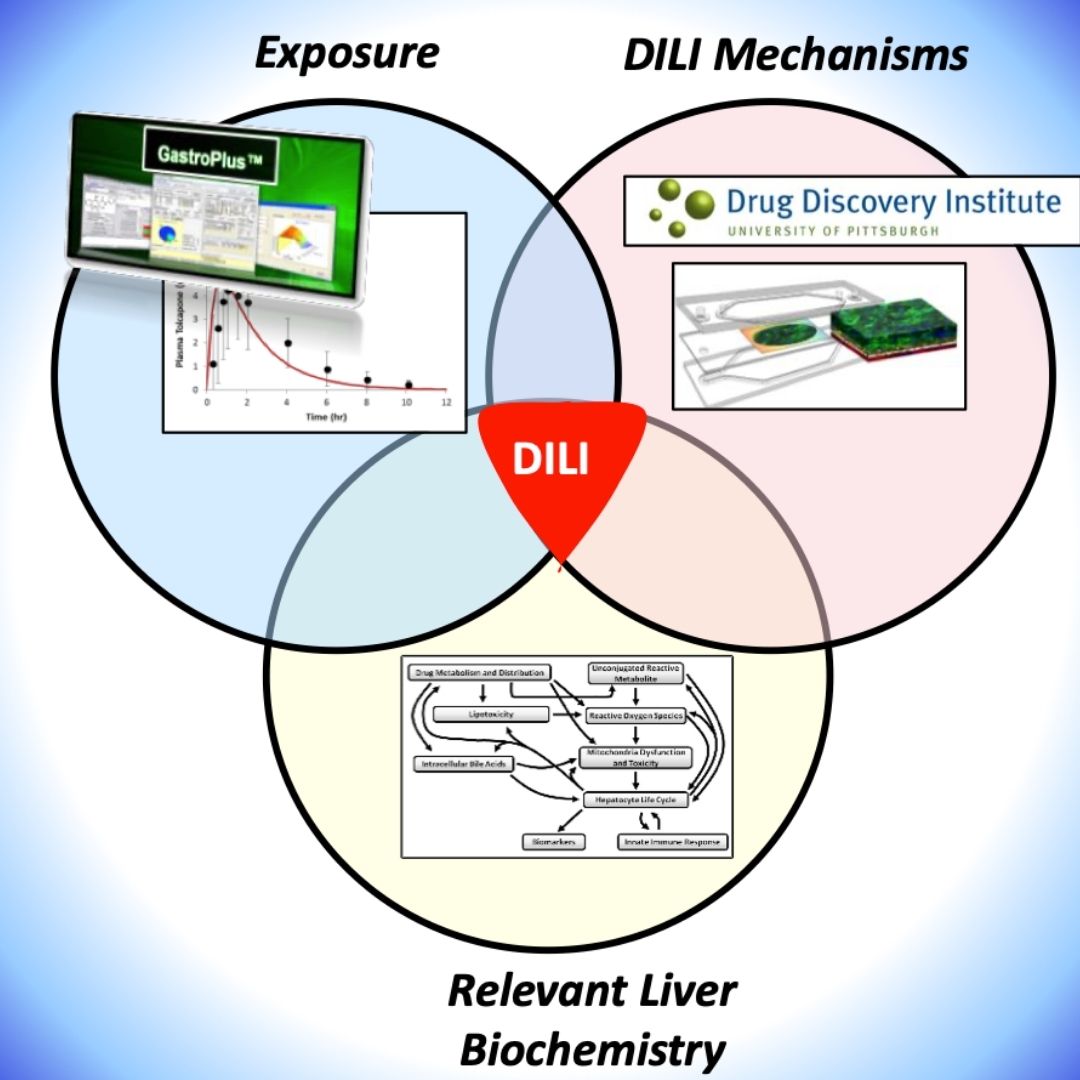
Integrating Human Biomimetic Liver Microphysiology System with Quantitative Systems Toxicology Modeling to Predict DILI
Integrating Human Biomimetic Liver Microphysiology System with Quantitative Systems Toxicology Modeling to Predict DILI
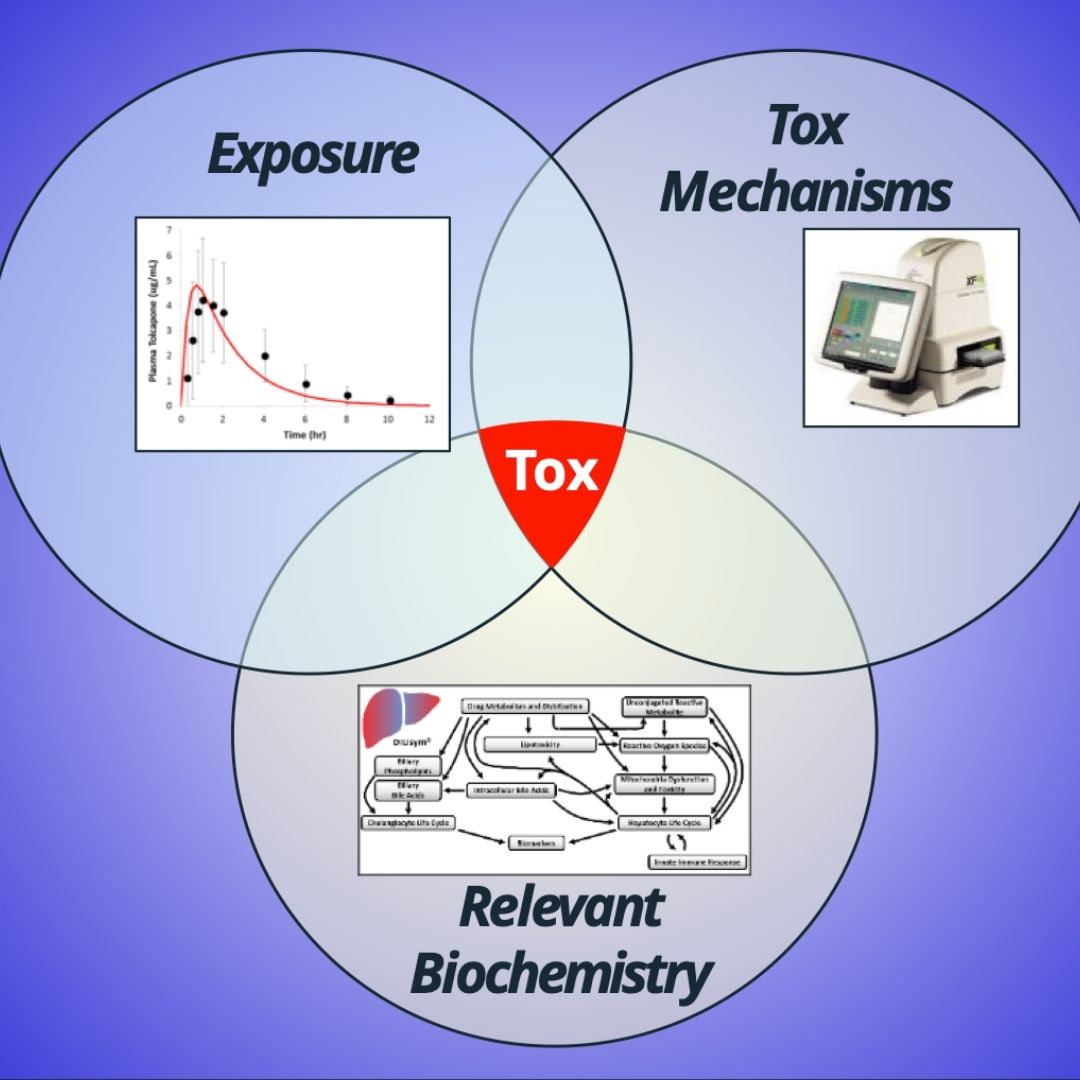
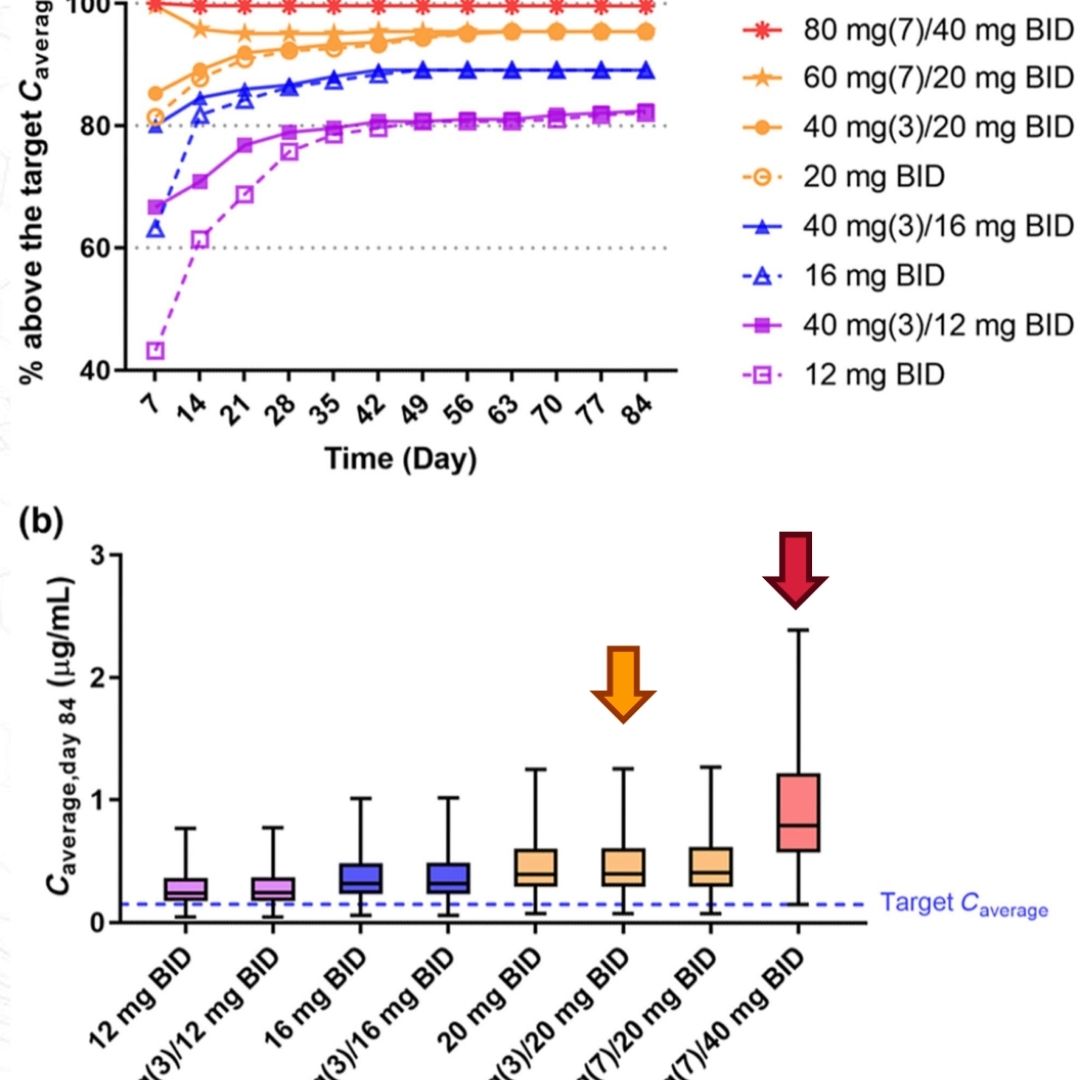
Construction of a Simulated Population of Post-Menopausal Women for the Prediction of Drug-Induced Liver Injury (DILI)
ASCPT 2024: Construction of a Simulated Population of Post-Menopausal Women for the Prediction of Drug-Induced Liver Injury (DILI)
Lunch & Learn – SOT 63rd Annual Meeting and ToxExpo
SOT 2024: What's New at Simulations Plus, Your Partner in Winning, for Exposure and Safety Assessment
Simulations Plus Investor Day 2023
Putting Clients First Drives Growth and Fuels Innovation
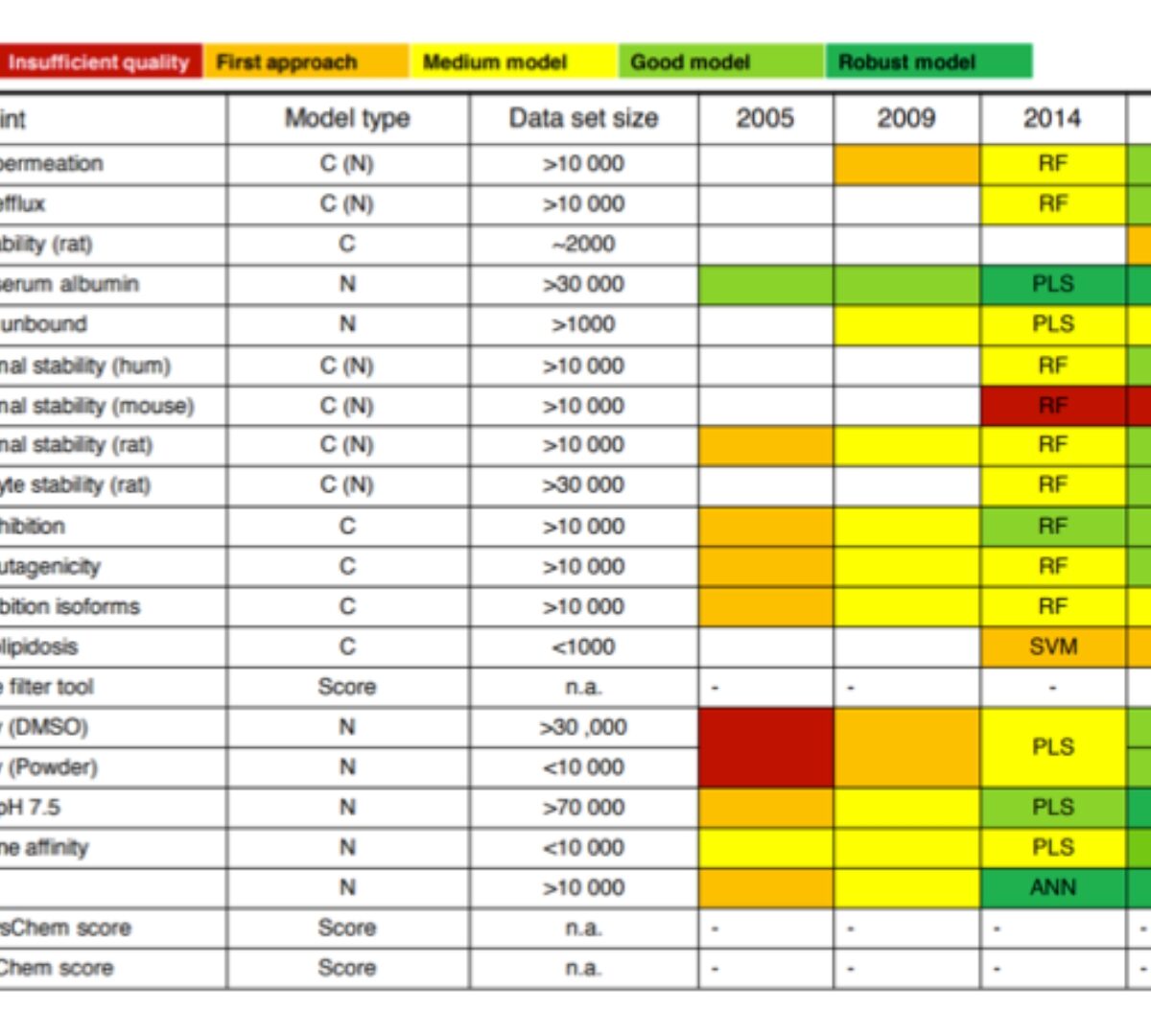
Contributed data, collaboration and experience with ionization models – Part 1. Bayer Pharmaceuticals
World‘s best in class pKa prediction tool
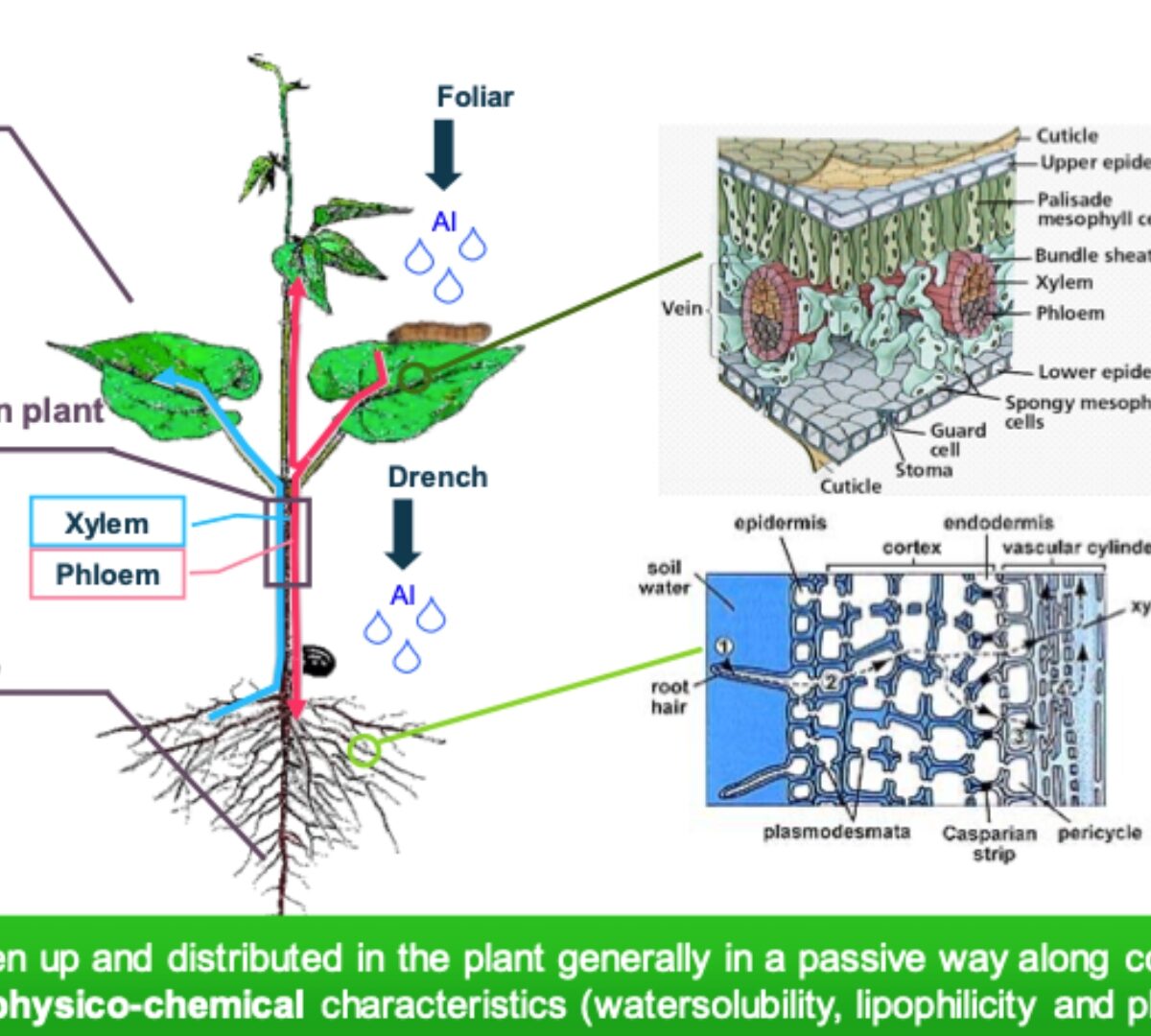
Utilizing pKa Values for Effective Crop Protection: On Systemicity and Efficacy of Molecules
pKa important factor in research screening cascade of molecules