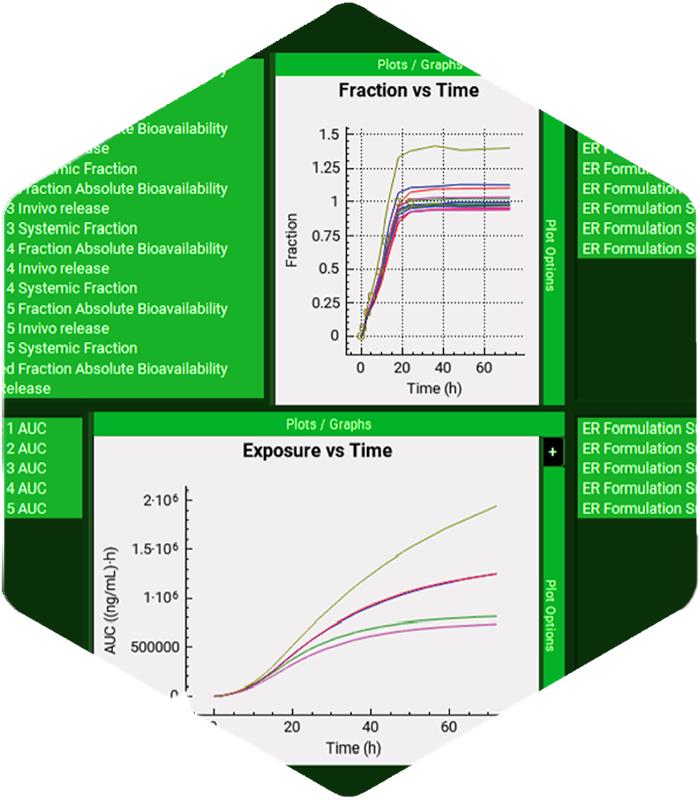
As a researcher in the life sciences space, you’re expected to make informed decisions as quickly as possible. A wrong one sets you back severely, both in terms of time, money, and getting to market. Now, more than ever, it is important to consider incorporating new technology across your company – like PBBM & PBPK modeling.